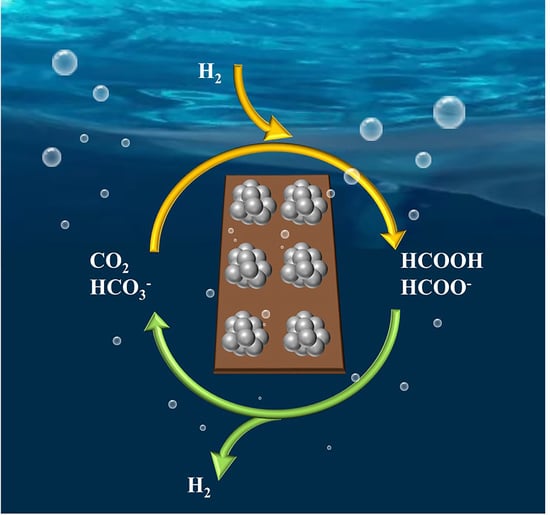
Application of Heterogeneous Catalysis in Formic Acid-Based Hydrogen Cycle System
Abstract
:1. Introduction
2. Heterogeneous Catalysis in FA-based Hydrogen Cycle System
2.1. Monometal-Based Heterogeneous Catalytic System
2.1.1. Activated Carbon-Supported Monometallic Catalysts
2.1.2. Mesoporous Carbon-Supported Monometallic Catalysts
2.1.3. Graphite-Supported Monometallic Catalysts
2.1.4. Mesoporous Graphitic Carbon Nitride-Supported Monometallic Catalysts
2.2. Bimetal-Based Heterogeneous Catalytic System
2.2.1. Molecular Sieve-Supported Bimetallic Catalysts
2.2.2. Zeolite-Supported Bimetallic Catalysts
2.2.3. Activated Carbon-Supported Bimetallic Catalysts
2.2.4. Mesoporous Carbon-Supported Bimetallic Catalysts
2.2.5. Graphene-Supported Bimetallic Catalysts
2.2.6. Metal Oxide-Supported Bimetallic Catalysts
3. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kar, S.; Rauch, M.; Leitus, G.; Ben-David, Y.; Milstein, D. Highly efficient additive-free dehydrogenation of neat formic acid. Nat. Catal. 2021, 4, 193–201. [Google Scholar] [CrossRef]
- Eppinger, J.; Huang, K.W. Formic Acid as a Hydrogen Energy Carrier. ACS Energy Lett. 2016, 2, 188–195. [Google Scholar] [CrossRef]
- Chen, H.; Shuang, H.; Lin, W.; Li, X.; Zhang, Z.; Li, J.; Fu, J. Tuning Interfacial Electronic Properties of Palladium Oxide on Vacancy-Abundant Carbon Nitride for Low-Temperature Dehydrogenation. ACS Catal. 2021, 11, 6193–6199. [Google Scholar] [CrossRef]
- Wei, D.; Sang, R.; Sponholz, P.; Junge, H.; Beller, M. Reversible hydrogenation of carbon dioxide to formic acid using a Mn-pincer complex in the presence of lysine. Nat. Energy 2022, 7, 438–447. [Google Scholar] [CrossRef]
- Park, K.; Gunasekar, G.H.; Kim, S.H.; Park, H.; Kim, S.; Park, K.; Jung, K.D.; Yoon, S. CO2 hydrogenation to formic acid over heterogenized ruthenium catalysts using a fixed bed reactor with separation units. Green Chem. 2020, 22, 1639–1649. [Google Scholar] [CrossRef]
- Onishi, N.; Iguchi, M.; Yang, X.; Kanega, R.; Kawanami, H.; Xu, Q.; Himeda, Y. Development of Effective Catalysts for Hydrogen Storage Technology Using Formic Acid. Adv. Energy Mater. 2018, 9, 1801275. [Google Scholar] [CrossRef]
- Amos, R.I.J.; Heinroth, F.; Chan, B.; Ward, A.J.; Zheng, S.; Haynes, B.S.; Easton, C.J.; Masters, A.F.; Maschmeyer, T.; Radom, L. Hydrogen from Formic Acid via Its Selective Disproportionation over Nanodomain-Modified Zeolites. ACS Catal. 2015, 5, 4353–4362. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, S.; Hou, M.; Yang, G.; Han, B. Continuous-flow formic acid production from the hydrogenation of CO2 without any base. Green Chem. 2021, 23, 1978–1982. [Google Scholar] [CrossRef]
- Graetz, J. New approaches to hydrogen storage. Chem. Soc. Rev. 2009, 38, 73–82. [Google Scholar] [CrossRef]
- Murray, L.J.; Dinca, M.; Long, J.R. Hydrogen storage in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef]
- Sousa-Castillo, A.; Li, F.; Carbo-Argibay, E.; Correa-Duarte, M.A.; Klinkova, A. Pd-CNT-SiO2 nanoskein: Composite structure design for formic acid dehydrogenation. Chem. Commun. 2019, 55, 10733–10736. [Google Scholar] [CrossRef]
- Liu, H.; Lei, Q.; Miao, R.; Sun, M.; Qin, C.; Zhang, L.; Ye, G.; Yao, Y.; Huang, B.; Ma, Z. Asymmetric Coordination of Single-Atom Co Sites Achieves Efficient Dehydrogenation Catalysis. Adv. Funct. Mater. 2022, 32, 220408. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, S.; Meng, X.; Mao, S.; Lian, X.; Wang, Y. Ultrasmall PdAu alloy nanoparticles anchored on amine-functionalized hierarchically porous carbon as additive-free catalysts for highly efficient dehydrogenation of formic acid. Appl. Catal. B 2021, 291, 120140. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Mao, S.; Gong, Y.; Chen, Y.; Wang, Y. Pd nanoparticles anchored on amino-functionalized hierarchically porous carbon for efficient dehydrogenation of formic acid under ambient conditions. J. Mater. Chem. A 2019, 7, 25791–25795. [Google Scholar] [CrossRef]
- Yan, J.M.; Li, S.J.; Yi, S.S.; Wulan, B.R.; Zheng, W.T.; Jiang, Q. Anchoring and Upgrading Ultrafine NiPd on Room-Temperature-Synthesized Bifunctional NH2-N-rGO toward Low-Cost and Highly Efficient Catalysts for Selective Formic Acid Dehydrogenation. Adv. Mater. 2018, 30, 1703038. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Liu, X.; Guo, Y.; Pao, C.W.; Chen, J.L.; Hu, Y.; Wang, Y. NiAl2O4 Spinel Supported Pt Catalyst: High Performance and Origin in Aqueous-Phase Reforming of Methanol. ACS Catal. 2019, 9, 9671–9682. [Google Scholar] [CrossRef]
- Chen, L.N.; Hou, K.P.; Liu, Y.S.; Qi, Z.Y.; Zheng, Q.; Lu, Y.H.; Chen, J.Y.; Chen, J.L.; Pao, C.W.; Wang, S.B.; et al. Efficient Hydrogen Production from Methanol Using a Single-Site Pt1/CeO2 Catalyst. J. Am. Chem. Soc. 2019, 141, 17995–17999. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, W.; Gao, R.; Yao, S.; Zhang, X.; Xu, W.; Zheng, S.; Jiang, Z.; Yu, Q.; Li, Y.W.; et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 2017, 544, 80–83. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, Q.; Zhang, T.; Zhang, J.; Dong, Z.; Ma, Y.; Wu, Z.; Wang, H.; Bao, X.; Sun, Q.; et al. Cobalt-Promoted Noble-Metal Catalysts for Efficient Hydrogen Generation from Ammonia Borane Hydrolysis. J. Am. Chem. Soc. 2023, 145, 5486–5495. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, W.; Feng, D.; Xu, H.; Qin, Y. Porous titania nanotube confined ultrafine platinum catalysts synthesized by atomic layer deposition with enhanced hydrolytic dehydrogenation performance. Appl. Catal. B 2022, 312, 121405. [Google Scholar] [CrossRef]
- Guan, S.; Liu, Y.; Zhang, H.; Wei, H.; Liu, T.; Wu, X.; Wen, H.; Shen, R.; Mehdi, S.; Ge, X.; et al. Atomic Interface-Exciting Catalysis on Cobalt Nitride-Oxide for Accelerating Hydrogen Generation. Small 2022, 18, e2107417. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, Q.; Shi, R.; Huang, M.; Lu, Z.H. A step-growth strategy to grow vertical porous aromatic framework nanosheets on graphene oxide: Hybrid material-confined Co for ammonia borane methanolysis. Carbon Energy 2023, e3572023. [Google Scholar]
- Xu, F.; Wang, Y.; Wang, C.; Huang, W.; Liu, X. Dehydrogenation of hydrous hydrazine over carbon nanosphere- supported PtNi nanoparticles for on-demand H2 release. Fuel 2023, 332, 126116. [Google Scholar] [CrossRef]
- Wang, C.; Astruc, D. Recent developments of nanocatalyzed liquid-phase hydrogen generation. Chem. Soc. Rev. 2021, 50, 3437–3484. [Google Scholar] [PubMed]
- Luo, F.; Miao, X.; Chu, W.; Wu, P.; Tong, D.G. Retracted Article: Preparation of face-centered-cubic indium nanocubes and their superior dehydrogenation activity towards aqueous hydrazine with the assistance of light. J. Mater. Chem. A 2016, 4, 17665–17672. [Google Scholar]
- Zhang, Y.; Lyu, Y.; Wang, Y.; Li, C.; Jiang, M.; Ding, Y. Highly active and stable porous polymer heterogenous catalysts for decomposition of formic acid to produce H2. Chin. J. Catal. 2019, 40, 147–151. [Google Scholar] [CrossRef]
- Qin, X.; Li, H.; Xie, S.; Li, K.; Jiang, T.; Ma, X.Y.; Jiang, K.; Zhang, Q.; Terasaki, O.; Wu, Z.; et al. Mechanistic Analysis-Guided Pd-Based Catalysts for Efficient Hydrogen Production from Formic Acid Dehydrogenation. ACS Catal. 2020, 10, 3921–3932. [Google Scholar] [CrossRef]
- Yang, X.; Xu, Q. Gold-containing metal nanoparticles for catalytic hydrogen generation from liquid chemical hydrides. Chin. J. Catal. 2016, 37, 1594–1599. [Google Scholar]
- Hou, T.; Luo, Q.; Li, Q.; Zu, H.; Cui, P.; Chen, S.; Lin, Y.; Chen, J.; Zheng, X.; Zhu, W.; et al. Modulating oxygen coverage of Ti3C2Tx MXenes to boost catalytic activity for HCOOH dehydrogenation. Nat. Commun. 2020, 11, 4251. [Google Scholar] [CrossRef]
- Shao, X.; Yang, X.; Xu, J.; Liu, S.; Miao, S.; Liu, X.; Su, X.; Duan, H.; Huang, Y.; Zhang, T. Iridium Single-Atom Catalyst Performing a Quasi-homogeneous Hydrogenation Transformation of CO2 to Formate. Chem 2019, 5, 693–705. [Google Scholar] [CrossRef]
- Akbayrak, S.; Tonbul, Y.; Özkar, S. Nanoceria supported palladium(0) nanoparticles: Superb catalyst in dehydrogenation of formic acid at room temperature. Appl. Catal. B 2017, 206, 384–392. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, Z.; Zhang, X.; Li, P.; Huang, Y.; Luo, X.; Liang, Z. Amine-functionalized sepiolite: Toward highly efficient palladium nanocatalyst for dehydrogenation of additive-free formic acid. Int. J. Hydrogen Energy 2019, 44, 16707–16717. [Google Scholar] [CrossRef]
- Zhang, S.; Qian, Y.; Ahn, W. Catalytic dehydrogenation of formic acid over palladium nanoparticles immobilized on fibrous mesoporous silica KCC-1. Chin. J. Catal. 2019, 40, 1704–1712. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, H.; Cao, D.; Zeng, X.C.; Cheng, D. Hydrogen Production via Efficient Formic Acid Decomposition: Engineering the Surface Structure of Pd-Based Alloy Catalysts by Design. ACS Catal. 2018, 9, 781–790. [Google Scholar] [CrossRef]
- Zhai, S.; Jiang, S.; Liu, C.; Li, Z.; Yu, T.; Sun, L.; Ren, G.; Deng, W. Liquid Sunshine: Formic Acid. J. Phys. Chem. Lett. 2022, 13, 8586–8600. [Google Scholar] [CrossRef]
- Li, Z.; Yang, X.; Tsumori, N.; Liu, Z.; Himeda, Y.; Autrey, T.; Xu, Q. Tandem Nitrogen Functionalization of Porous Carbon: Toward Immobilizing Highly Active Palladium Nanoclusters for Dehydrogenation of Formic Acid. ACS Catal. 2017, 7, 2720–2724. [Google Scholar] [CrossRef]
- Han, L.; Zhang, L.; Wu, H.; Zu, H.; Cui, P.; Guo, J.; Guo, R.; Ye, J.; Zhu, J.; Zheng, X.; et al. Anchoring Pt Single Atoms on Te Nanowires for Plasmon-Enhanced Dehydrogenation of Formic Acid at Room Temperature. Adv. Sci. 2019, 6, 1900006. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dong, M.; Liu, S.; Xie, Z.; Yang, J.; Li, S.; Wang, Y.; Du, J.; Liu, H.; Han, B. CO2 Hydrogenation to Formate Catalyzed by Ru Coordinated with a N,P-Containing Polymer. ACS Catal. 2020, 10, 8557–8566. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, Y.; Li, W.; Hu, S.; Song, J.; Jiang, T.; Han, B. Hydrogenation of carbon dioxide is promoted by a task-specific ionic liquid. Angew. Chem. Int. Ed. 2008, 47, 1127–1129. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, X.; Li, L.; Miao, S.; Li, Y.; Li, Y.; Wang, X.; Huang, Y.; Zhang, T. Direct catalytic hydrogenation of CO2 to formate over a Schiff-base-mediated gold nanocatalyst. Nat. Commun. 2017, 8, 1407. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Santos, S.; Urbina-Blanco, C.A.; Hernández, W.Y.; Impéror-Clerc, M.; Vovk, E.I.; Marinova, M.; Ersen, O.; Baaziz, W.; Safonova, O.V.; et al. Solid micellar Ru single-atom catalysts for the water-free hydrogenation of CO2 to formic acid. Appl. Catal. B 2021, 290, 120036. [Google Scholar] [CrossRef]
- Li, Z.; Rayder, T.M.; Luo, L.; Byers, J.A.; Tsung, C.K. Aperture-Opening Encapsulation of a Transition Metal Catalyst in a Metal-Organic Framework for CO2 Hydrogenation. J. Am. Chem. Soc. 2018, 140, 8082–8085. [Google Scholar] [CrossRef]
- Kim, E.H.; Lee, M.H.; Kim, J.; Ra, E.C.; Lee, J.H.; Lee, J.S. Synergy between single atoms and nanoclusters of Pd/g-C3N4 catalysts for efficient base-free CO2 hydrogenation to formic acid. Chin. J. Catal. 2023, 47, 214–221. [Google Scholar] [CrossRef]
- Zhou, Z.; Gao, P. Direct carbon dioxide hydrogenation to produce bulk chemicals and liquid fuels via heterogeneous catalysis. Chin. J. Catal. 2022, 43, 2045–2056. [Google Scholar] [CrossRef]
- Doustkhah, E.; Hasani, M.; Ide, Y.; Assadi, M.H.N. Pd Nanoalloys for H2 Generation from Formic Acid. ACS Appl. Nano Mater. 2019, 3, 22–43. [Google Scholar] [CrossRef]
- Hong, W.; Kitta, M.; Tsumori, N.; Himeda, Y.; Autrey, T.; Xu, Q. Immobilization of highly active bimetallic PdAu nanoparticles onto nanocarbons for dehydrogenation of formic acid. J. Mater. Chem. A 2019, 7, 18835–18839. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Q.; Nie, W.; Yao, Q.; Zhang, Z.; Lu, Z.H. Anchoring IrPdAu Nanoparticles on NH2-SBA-15 for Fast Hydrogen Production from Formic Acid at Room Temperature. ACS Appl. Mater. Interfaces 2020, 12, 8082–8090. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Sun, Q.; Bai, R.; Li, X.; Guo, G.; Yu, J. In Situ Confinement of Ultrasmall Pd Clusters within Nanosized Silicalite-1 Zeolite for Highly Efficient Catalysis of Hydrogen Generation. J. Am. Chem. Soc. 2016, 138, 7484–7487. [Google Scholar] [CrossRef]
- Li, S.J.; Zhou, Y.T.; Kang, X.; Liu, D.X.; Gu, L.; Zhang, Q.H.; Yan, J.M.; Jiang, Q. A Simple and Effective Principle for a Rational Design of Heterogeneous Catalysts for Dehydrogenation of Formic Acid. Adv. Mater. 2019, 31, e1806781. [Google Scholar] [CrossRef]
- Moret, S.; Dyson, P.J.; Laurenczy, G. Direct synthesis of formic acid from carbon dioxide by hydrogenation in acidic media. Nat. Commun. 2014, 5, 4017. [Google Scholar] [CrossRef]
- Mori, K.; Sano, T.; Kobayashi, H.; Yamashita, H. Surface Engineering of a Supported PdAg Catalyst for Hydrogenation of CO2 to Formic Acid: Elucidating the Active Pd Atoms in Alloy Nanoparticles. J. Am. Chem. Soc. 2018, 140, 8902–8909. [Google Scholar] [CrossRef]
- Mellmann, D.; Sponholz, P.; Junge, H.; Beller, M. Formic acid as a hydrogen storage material—Development of homogeneous catalysts for selective hydrogen release. Chem. Soc. Rev. 2016, 45, 3954–3988. [Google Scholar] [CrossRef] [PubMed]
- Hull, J.F.; Himeda, Y.; Wang, W.H.; Hashiguchi, B.; Periana, R.; Szalda, D.J.; Muckerman, J.T.; Fujita, E. Reversible hydrogen storage using CO2 and a proton-switchable iridium catalyst in aqueous media under mild temperatures and pressures. Nat. Chem. 2012, 4, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xu, Y.; Meng, Y.; Wang, L.; Wang, H.; Huang, Y.; Onishi, N.; Wang, L.; Fan, Z.; Himeda, Y. Heterogeneous Catalysis for Carbon Dioxide Mediated Hydrogen Storage Technology Based on Formic Acid. Adv. Energy Mater. 2022, 12, 2200817. [Google Scholar] [CrossRef]
- Mori, K.; Taga, T.; Yamashita, H. Isolated Single-Atomic Ru Catalyst Bound on a Layered Double Hydroxide for Hydrogenation of CO2 to Formic Acid. ACS Catal. 2017, 7, 3147–3151. [Google Scholar] [CrossRef]
- Bulut, A.; Yurderi, M.; Karatas, Y.; Say, Z.; Kivrak, H.; Kaya, M.; Gulcan, M.; Ozensoy, E.; Zahmakiran, M. MnOx-Promoted PdAg Alloy Nanoparticles for the Additive-Free Dehydrogenation of Formic Acid at Room Temperature. ACS Catal. 2015, 5, 6099–6110. [Google Scholar] [CrossRef]
- Karatas, Y.; Bulut, A.; Yurderi, M.; Ertas, I.E.; Alal, O.; Gulcan, M.; Celebi, M.; Kivrak, H.; Kaya, M.; Zahmakiran, M. PdAu-MnO nanoparticles supported on amine-functionalized SiO2 for the room temperature dehydrogenation of formic acid in the absence of additives. Appl. Catal. B 2016, 180, 586–595. [Google Scholar] [CrossRef]
- Zou, L.; Chen, M.; Zhang, Q.; Mao, Q.; Huang, Y.; Liang, Z. Pd/UIO-66/sepiolite: Toward highly efficient dual-supported Pd-based catalyst for dehydrogenation of formic acid at room temperature. J. Catal. 2020, 388, 66–76. [Google Scholar] [CrossRef]
- Sordakis, K.; Tang, C.; Vogt, L.K.; Junge, H.; Dyson, P.J.; Beller, M.; Laurenczy, G. Homogeneous Catalysis for Sustainable Hydrogen Storage in Formic Acid and Alcohols. Chem. Rev. 2018, 118, 372–433. [Google Scholar] [CrossRef]
- Jantke, D.; Pardatscher, L.; Drees, M.; Cokoja, M.; Herrmann, W.A.; Kuhn, F.E. Hydrogen Production and Storage on a Formic Acid/Bicarbonate Platform using Water-Soluble N-Heterocyclic Carbene Complexes of Late Transition Metals. ChemSusChem 2016, 9, 2849–2854. [Google Scholar] [CrossRef]
- Wang, J.; Jin, H.; Wang, W.H.; Zhao, Y.; Li, Y.; Bao, M. Ultrasmall Ni-ZnO/SiO2 Synergistic Catalyst for Highly Efficient Hydrogenation of NaHCO3 to Formic Acid. ACS Appl. Mater. Interfaces 2020, 12, 19581–19586. [Google Scholar] [CrossRef]
- Treigerman, Z.; Sasson, Y. Generation and Quantification of Formate Ion Produced from Aqueous Sodium Bicarbonate in the Presence of Homogeneous Ruthenium Catalyst. ACS Omega 2018, 3, 12797–12801. [Google Scholar] [CrossRef]
- Wang, Q.; Tsumori, N.; Kitta, M.; Xu, Q. Fast Dehydrogenation of Formic Acid over Palladium Nanoparticles Immobilized in Nitrogen-Doped Hierarchically Porous Carbon. ACS Catal. 2018, 8, 12041–12045. [Google Scholar] [CrossRef]
- Lundsted, L.G. The Hydrogenation of Sodium Bicarbonate to Sodium Formate. J. Am. Chem. Soc. 1949, 71, 323–324. [Google Scholar] [CrossRef]
- Xu, F.; Huang, W.; Wang, Y.; Astruc, D.; Liu, X. Efficient and controlled H2 release from sodium formate. Inorg. Chem. Front. 2022, 9, 3514–3521. [Google Scholar] [CrossRef]
- Xu, F.; Yan, J.; Wang, Y.; Liu, X. Mechanistic insight into efficient H2 generation upon HCOONa hydrolysis. iScience 2023, 26, 106504. [Google Scholar] [CrossRef]
- Grasemann, M.; Laurenczy, G. Formic acid as a hydrogen source—Recent developments and future trends. Energy Environ. Sci. 2012, 5, 8171–8181. [Google Scholar] [CrossRef]
- Wang, W.H.; Himeda, Y.; Muckerman, J.T.; Manbeck, G.F.; Fujita, E. CO2 Hydrogenation to Formate and Methanol as an Alternative to Photo- and Electrochemical CO2 Reduction. Chem. Rev. 2015, 115, 12936–12973. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Xu, Q.; Yu, J. Nanopore-Supported Metal Nanocatalysts for Efficient Hydrogen Generation from Liquid-Phase Chemical Hydrogen Storage Materials. Adv. Mater. 2020, 32, 2001818. [Google Scholar] [CrossRef]
- Alvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef]
- Wei, D.; Shi, X.; Qu, R.; Junge, K.; Junge, H.; Beller, M. Toward a Hydrogen Economy: Development of Heterogeneous Catalysts for Chemical Hydrogen Storage and Release Reactions. ACS Energy Lett. 2022, 7, 3734–3752. [Google Scholar] [CrossRef]
- Chatterjee, S.; Dutta, I.; Lum, Y.; Lai, Z.; Huang, K.W. Enabling storage and utilization of low-carbon electricity: Power to formic acid. Energy Environ. Sci. 2021, 14, 1194–1246. [Google Scholar] [CrossRef]
- Asefa, T.; Koh, K.; Yoon, C.W. CO2-Mediated H2 Storage-Release with Nanostructured Catalysts: Recent Progresses, Challenges, and Perspectives. Adv. Energy Mater. 2019, 9, 1901158. [Google Scholar] [CrossRef]
- Ji, L.; Cui, T.; Nie, X.; Zheng, Y.; Zheng, X.; Fu, H.; Yuan, M.; Chen, H.; Xu, J.; Li, R. Catalytic hydrogenation of CO2 with unsymmetric N-heterocyclic carbene–nitrogen–phosphine ruthenium complexes. Catal. Sci. Technol. 2021, 11, 6965–6969. [Google Scholar] [CrossRef]
- Kipshagen, A.; Baums, J.C.; Hartmann, H.; Besmehn, A.; Hausoul, P.J.C.; Palkovits, R. Formic acid as H2 storage system: Hydrogenation of CO2 and decomposition of formic acid by solid molecular phosphine catalysts. Catal. Sci. Technol. 2022, 12, 5649–5656. [Google Scholar] [CrossRef]
- Park, K.; Gunasekar, G.H.; Prakash, N.; Jung, K.D.; Yoon, S. A Highly Efficient Heterogenized Iridium Complex for the Catalytic Hydrogenation of Carbon Dioxide to Formate. ChemSusChem 2015, 8, 3410–3413. [Google Scholar] [CrossRef]
- Wang, H.; Chi, Y.; Gao, D.; Wang, Z.; Wang, C.; Wang, L.; Wang, M.; Cheng, D.; Zhang, J.; Wu, C.; et al. Enhancing formic acid dehydrogenation for hydrogen production with the metal/organic interface. Appl. Catal. B 2019, 255, 117776. [Google Scholar] [CrossRef]
- Sun, R.; Liao, Y.; Bai, S.T.; Zheng, M.; Zhou, C.; Zhang, T.; Sels, B.F. Heterogeneous catalysts for CO2 hydrogenation to formic acid/formate: From nanoscale to single atom. Energy Environ. Sci. 2021, 14, 1247–1285. [Google Scholar] [CrossRef]
- Gunasekar, G.H.; Yoon, S. A phenanthroline-based porous organic polymer for the iridium-catalyzed hydrogenation of carbon dioxide to formate. J. Mater. Chem. A 2019, 7, 14019–14026. [Google Scholar] [CrossRef]
- Gunasekar, G.H.; Shin, J.; Jung, K.D.; Park, K.; Yoon, S. Design Strategy toward Recyclable and Highly Efficient Heterogeneous Catalysts for the Hydrogenation of CO2 to Formate. ACS Catal. 2018, 8, 4346–4353. [Google Scholar] [CrossRef]
- Shen, Y.; Zhan, Y.; Bai, C.; Ning, F.; Wang, H.; Wei, J.; Lv, G.; Zhou, X. Immobilized iridium complexes for hydrogen evolution from formic acid dehydrogenation. Sustain. Energy Fuels 2020, 4, 2519–2526. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Mao, S.; Chen, Z.; Wang, Y. Coordination environment of active sites and their effect on catalytic performance of heterogeneous catalysts. Chin. J. Catal. 2022, 43, 928–955. [Google Scholar] [CrossRef]
- Zaidman, B.; Wiener, H.; Sasson, Y. Formate salts as chemical carriers in hydrogen storage and transportation. Int. J. Hydrogen Energy 1986, 11, 341–347. [Google Scholar] [CrossRef]
- Wiener, H.; Zaidman, B.; Sasson, Y. Storage of energy by solutions of alkali formate salts. Sol. Energy 1989, 43, 291–296. [Google Scholar] [CrossRef]
- Su, J.; Yang, L.; Lu, M.; Lin, H. Highly efficient hydrogen storage system based on ammonium bicarbonate/formate redox equilibrium over palladium nanocatalysts. ChemSusChem 2015, 8, 813–816. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, J.; Yao, Y.; Sun, J.; Wang, Y.; Lin, H. Renewable energy storage via efficient reversible hydrogenation of piperidine captured CO2. Green Chem. 2018, 20, 4292–4298. [Google Scholar] [CrossRef]
- Bi, Q.Y.; Lin, J.D.; Liu, Y.M.; He, H.Y.; Huang, F.Q.; Cao, Y. Dehydrogenation of Formic Acid at Room Temperature: Boosting Palladium Nanoparticle Efficiency by Coupling with Pyridinic-Nitrogen-Doped Carbon. Angew. Chem. Int. Ed. 2016, 55, 11849–11853. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, J.; Shao, X.; Su, X.; Huang, Y.; Zhang, T. Palladium on Nitrogen-Doped Mesoporous Carbon: A Bifunctional Catalyst for Formate-Based, Carbon-Neutral Hydrogen Storage. ChemSusChem 2016, 9, 246–251. [Google Scholar] [CrossRef]
- Koh, K.; Jeon, M.; Chevrier, D.M.; Zhang, P.; Yoon, C.W.; Asefa, T. Novel nanoporous N-doped carbon-supported ultrasmall Pd nanoparticles: Efficient catalysts for hydrogen storage and release. Appl. Catal. B 2017, 203, 820–828. [Google Scholar] [CrossRef]
- Shao, X.; Miao, X.; Zhang, T.; Wang, W.; Wang, J.; Ji, X. Pd Nanoparticles Supported on N- and P-Co-doped Carbon as Catalysts for Reversible Formate-Based Chemical Hydrogen Storage. ACS Appl. Nano Mater. 2020, 3, 9209–9217. [Google Scholar] [CrossRef]
- Bi, Q.Y.; Lin, J.D.; Liu, Y.M.; Du, X.L.; Wang, J.Q.; He, H.Y.; Cao, Y. An aqueous rechargeable formate-based hydrogen battery driven by heterogeneous Pd catalysis. Angew. Chem. Int. Ed. 2014, 53, 13583–13587. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Kim, M.S.; Kwon, J.A.; Shin, Y.J.; Yoon, C.W.; Lim, D.H. Fundamental Mechanisms of Reversible Dehydrogenation of Formate on N-Doped Graphene-Supported Pd Nanoparticles. J. Phys. Chem. C 2018, 123, 1539–1549. [Google Scholar] [CrossRef]
- Lee, J.H.; Ryu, J.; Kim, J.Y.; Nam, S.W.; Han, J.H.; Lim, T.H.; Gautam, S.; Chae, K.H.; Yoon, C.W. Carbon dioxide mediated, reversible chemical hydrogen storage using a Pd nanocatalyst supported on mesoporous graphitic carbon nitride. J. Mater. Chem. A 2014, 2, 9490–9495. [Google Scholar] [CrossRef]
- Shao, X.; Xu, J.; Huang, Y.; Su, X.; Duan, H.; Wang, X.; Zhang, T. Pd@C3N4 nanocatalyst for highly efficient hydrogen storage system based on potassium bicarbonate/formate. AlChE J. 2016, 62, 2410–2418. [Google Scholar] [CrossRef]
- Mori, K.; Masuda, S.; Tanaka, H.; Yoshizawa, K.; Che, M.; Yamashita, H. Phenylamine-functionalized mesoporous silica supported PdAg nanoparticles: A dual heterogeneous catalyst for formic acid/CO2-mediated chemical hydrogen delivery/storage. Chem. Commun. 2017, 53, 4677–4680. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Chen, B.W.J.; Wang, N.; He, Q.; Chang, A.; Yang, C.M.; Asakura, H.; Tanaka, T.; Hulsey, M.J.; Wang, C.H.; et al. Zeolite-Encaged Pd-Mn Nanocatalysts for CO2 Hydrogenation and Formic Acid Dehydrogenation. Angew. Chem. Int. Ed. 2020, 59, 20183–20191. [Google Scholar] [CrossRef]
- Nakajima, K.; Tominaga, M.; Waseda, M.; Miura, H.; Shishido, T. Highly Efficient Supported Palladium–Gold Alloy Catalysts for Hydrogen Storage Based on Ammonium Bicarbonate/Formate Redox Cycle. ACS Sustain. Chem. Eng. 2019, 7, 6522–6530. [Google Scholar] [CrossRef]
- Zou, L.; Liu, Q.; Zhang, Q.; Zhu, Z.; Huang, Y.; Liang, Z. Synthesis of Bimetallic Pd-Based/Activated Carbon Catalyst by Biomass-Reduction Method for Highly Efficient Hydrogen Storage System Based on CO2/Formate. Ind. Eng. Chem. Res. 2022, 61, 2455–2468. [Google Scholar] [CrossRef]
- Jiang, S.; Yang, J.; Zhai, S.; Zhang, L.; Tu, R.; Yu, T.; Zhai, D.; Sun, L.; Deng, W.; Ren, G. Ambient Hydrogen Storage and Release Using CO2 and an L-Arginine-Functionalized PdAu Catalyst via pH Control. ACS Catal. 2022, 12, 14113–14122. [Google Scholar] [CrossRef]
- Masuda, S.; Mori, K.; Futamura, Y.; Yamashita, H. PdAg Nanoparticles Supported on Functionalized Mesoporous Carbon: Promotional Effect of Surface Amine Groups in Reversible Hydrogen Delivery/Storage Mediated by Formic Acid/CO2. ACS Catal. 2018, 8, 2277–2285. [Google Scholar] [CrossRef]
- Zhong, H.; Iguchi, M.; Chatterjee, M.; Ishizaka, T.; Kitta, M.; Xu, Q.; Kawanami, H. Interconversion between CO2 and HCOOH under Basic Conditions Catalyzed by PdAu Nanoparticles Supported by Amine-Functionalized Reduced Graphene Oxide as a Dual Catalyst. ACS Catal. 2018, 8, 5355–5362. [Google Scholar] [CrossRef]
- Masuda, S.; Shimoji, Y.; Mori, K.; Kuwahara, Y.; Yamashita, H. Interconversion of Formate/Bicarbonate for Hydrogen Storage/Release: Improved Activity Following Sacrificial Surface Modification of a Ag@Pd/TiO2 Catalyst with a TiOx Shell. ACS Appl. Energy Mater. 2020, 3, 5819–5829. [Google Scholar] [CrossRef]













| Hydrogenation Reaction | Dehydrogenation Reaction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Catalyst | Substrate | Additive | pH2/pCO2 (MPa) | T (°C) | TOF (h−1) | Substrate | Additive | T (°C) | TOF (h−1) | Ref. |
| Pd/AC | 1 M NH4HCO3 | / | 2.75/0 | 20 | 118 | 1 M HCO2NH4 | / | 80 | 1132 | [85] |
| Pd/AC | CO2 a | 1 M piperidine | 2.76/0 | 30 | 5945 | 1 M FPA b | / | [86] | ||
| Pd/CN0.25 | CO2 | 5.7 M NEt3 | 3/3 | 100 | 1837 | 1 M HCOOH | / | 25 | 5530 | [87] |
| Pd/NMC | 4 M KHCO3 | / | 6/0 | 80 | 799 | 2 M HCOOK | / | 60 | 1118 | [88] |
| Pd/PDMC | 1 M NaHCO₃ | / | 4/0 | 80 | 68 | 1 M NaHCO2 | / | 80 | 2562 | [89] |
| Pd/N,P-C | 4 M KHCO3 | / | 8/0 | 80 | 2805 | 4 M KHCO2 | / | 80 | 3248 | [90] |
| Pd/r-GO | 4.8 M KHCO3 | / | 4/0 | 100 | 242 | 4.8 M HCOOK | / | 80 | 11,299 | [91] |
| Pd/mpg-C3N4 | CO2 | 1.4 M NEt3 | 2/2 | 150 | 4 | 1 M HCOOH | / | 25 | 144 | [93] |
| Pd/mpg-C3N4 | 4 M KHCO3 | / | 8/0 | 80 | 4076 | 4 M HCOOK | / | 80 | 511 | [94] |
| Catalyst Sample | Binding Energy (eV) | |
|---|---|---|
| Pd0 3d3/2 | Pd0 3d5/2 | |
| Pd/AC | 341.28 | 335.92 |
| Pd-Cu/AC | 341.18 | 335.86 |
| Pd-Au/AC | 341.06 | 335.73 |
| Hydrogenation Reaction | Dehydrogenation Reaction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Catalyst | Substrate | Additive | pH2/pCO2 (MPa) | T (°C) | TOF (h−1) | Substrate | Additive | T (°C) | TOF (h−1) | Ref. |
| PdAg/SBA-15-phenylamine | CO2 | 1 M NaHCO3 | 1/1 | 100 | 36 | 0.9 M HCCOH | 0.1 M HCOONa | 75 | 822 | [95] |
| PdMn0.6@S-1 | CO2 | 1.5 M NaOH | 2/2 | 80 | 2151 | 2 M HCOOH | / | 60 | 6860 | [96] |
| 10Au1Pd/AC | 1 M NH4HCO3 | / | 5/0 | 60 | 5820 | 1 M HCO2NH4 | / | 40 | 4200 | [97] |
| Pd-Au/AC | CO2 | 1.8 M NEt3 | 5/5 | 110 | 81 | 1 M HCOOH | 0.5 M NEt3 | 80 | 431 | [98] |
| Pd-Cu/AC | CO2 | 1.8 M NEt3 | 3.5/3.5 | 110 | 100 | 1 M HCOOH | 0.5 M NEt3 | 80 | 101 | |
| Pd1Au2/AC-LA | CO2 | 1 M NaHCO3 | 0.075/0.025 | 25 | 138 | 1 M HCOOH | / | 25 | 1760 | [99] |
| PdAg/amine-MSC | CO2 | 1 M NaHCO3 | 1/1 | 100 | 35 | 0.27 M HCOOH | 0.03 M HCOONa | 75 | 5638 | [100] |
| Pd0.50Au0.50/PDA-rGO | 0.5 M KHCO3 | / | 5/0 | 50 | / | 6 M HCOOK | / | 80 | 1630 | [101] |
| 8 M HCOOH | / | 80 | 6980 | |||||||
| Pd@Ag/TiO2 | NaHCO3 | / | 3/0 | 80 | 1568 | HCOONa | / | 75 | 20,578 | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Qian, J.; Sun, Z.; Zhang, Z.; He, M.; Chen, Q. Application of Heterogeneous Catalysis in Formic Acid-Based Hydrogen Cycle System. Catalysts 2023, 13, 1168. https://doi.org/10.3390/catal13081168
Wang Z, Qian J, Sun Z, Zhang Z, He M, Chen Q. Application of Heterogeneous Catalysis in Formic Acid-Based Hydrogen Cycle System. Catalysts. 2023; 13(8):1168. https://doi.org/10.3390/catal13081168
Chicago/Turabian StyleWang, Zhenzhen, Junfeng Qian, Zhonghua Sun, Zhihui Zhang, Mingyang He, and Qun Chen. 2023. "Application of Heterogeneous Catalysis in Formic Acid-Based Hydrogen Cycle System" Catalysts 13, no. 8: 1168. https://doi.org/10.3390/catal13081168
APA StyleWang, Z., Qian, J., Sun, Z., Zhang, Z., He, M., & Chen, Q. (2023). Application of Heterogeneous Catalysis in Formic Acid-Based Hydrogen Cycle System. Catalysts, 13(8), 1168. https://doi.org/10.3390/catal13081168