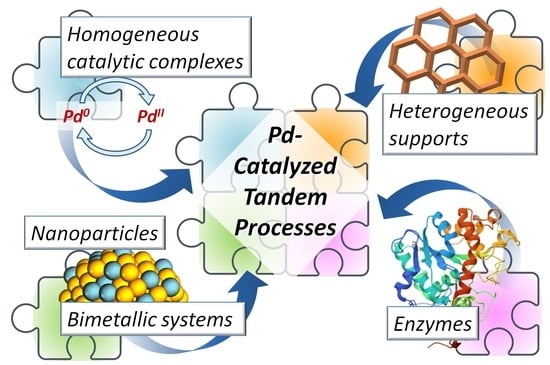
Recent Progress in Pd-Catalyzed Tandem Processes
Abstract
:1. Introduction
2. Pd-Catalyzed Homogeneous Tandem Catalysis
2.1. Coupling Tandem Processes
2.2. Isomerization Tandem Processes
2.3. Carbonylation Tandem Processes
2.4. Cyclization Tandem Processes
2.5. Other Tandem Processes
- (i)
- intramolecular hydroarylation of yanamides;
- (ii)
- hydroarylation-heterocyclization of 2-aminophenyl propiolate;
- (iii)
- Heck-heterocyclization;
- (iv)
- oxidative Heck-heterocyclization;
- (v)
- arylation of ortho-halo-cinnamides, followed by Buchwald-type intramolecular amidation.
3. Heterogenized PdComplexes in Tandem Processes
4. Heterogeneous Pd-Catalyzed Tandem Processes
- (i)
- size and shape selectivity, with active sites only accessible to substrates and intermediates with specific sizes and shapes;
- (ii)
- surface and solvent engineering that exploits differences in the hydrophobicity, hydrophilicity, and other properties of the catalizate’s components;
- (iii)
- metal site engineering through controlled size, exposed facets, composition, and their spatial distribution;
- (iv)
- reactor and process engineering (i.e., chemical looping, reactive separations, andmultiple sequential catalyst beds in flow reactors) [15].
5. Chemoenzymatic Processes
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Campos, J.F.; Berteina-Raboin, S. Tandem Catalysis: Synthesis of Nitrogen-Containing Heterocycles. Catalysts 2020, 10, 631. [Google Scholar] [CrossRef]
- Camp, J.E. Auto-Tandem Catalysis: Activation of Multiple, Mechanistically Distinct Process by a Single Catalyst. Eur. J. Org. Chem. 2017, 2017, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Fogg, D.E.; dos Santos, E.N. Tandem Catalysis: A Taxonomy and Illustrative Review. Coord. Chem. Rev. 2004, 248, 2365–2379. [Google Scholar] [CrossRef]
- Das, S.; Hong, D.; Chen, Z.; She, Z.; Hersh, W.H.; Subramaniam, G.; Chen, Y. Auto-Tandem Palladium Catalysis: From Isoxazoleto 2-Azafluorenone. Org. Lett. 2015, 17, 5578–5581. [Google Scholar] [CrossRef]
- Chen, P.; Chen, Z.-C.; Li, Y.; Ouyang, Q.; Du, W.; Chen, Y.-C. Auto-Tandem Cooperative Catalysis Using Phosphine/Palladium: Reactionof Morita–Baylis–Hillman Carbonates and Allylic Alcohols. Angew. Chem. 2019, 131, 4076–4080. [Google Scholar] [CrossRef]
- Alexander, J.R.; Shchepetkina, V.I.; Stankevich, K.S.; Benedict, R.J.; Bernhard, S.P.; Dreiling, R.J.; Cook, M.J. Pd-Catalyzed Rearrangement of N-Alloc-N-allyl Ynamides via Auto-Tandem Catalysis: Evidence for Reversible C–N Activation and Pd(0)-Accelerated Ketenimine Aza-Claisen Rearrangement. Org. Lett. 2021, 23, 559–564. [Google Scholar] [CrossRef]
- Jeske, K.; Rösler, T.; Belleflamme, M.; Rodenas, T.; Fischer, N.; Claeys, M.; Leitner, W.; Vorholt, A.J.; Prieto, G. Direct Conversion of Syngas to Higher Alcohols via Tandem Integration of Fischer–Tropsch Synthesis and Reductive Hydroformylation. Angew. Chem. Int. Ed. 2022, 61, e202201004. [Google Scholar] [CrossRef]
- Araújo, M.; MuñozCapdevila, I.; Díaz-Oltra, S.; Escuder, B. Tandem Catalysis of an Aldol-‘Click’ Reaction System within a Molecular Hydrogel. Molecules 2016, 21, 744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Thakur, V.K.; Chew, Y.M.J.; Li, S. Towards Next Generation “Smart” Tandem Catalysts with Sandwiched Mussel-inspired Layer Switch. Mater. Today Chem. 2020, 17, 100286. [Google Scholar] [CrossRef]
- Li, N.; Huang, B.; Dong, X.; Luo, J.; Wang, Y.; Wang, H.; Miao, D.; Pan, Y.; Jiao, F.; Xiao, J.; et al. Bifunctional Zeolites-silver Catalyst Enabled Tandem Oxidation of Formaldehyde at Low Temperatures. Nat. Commun. 2022, 13, 2209. [Google Scholar] [CrossRef]
- Gumus, I.; Ruzgar, A.; Karatas, Y.; Gülcan, M. Highly Efficient and Selective One-pot Tandem Imine Synthesis via Amine-alcohol Cross-coupling Reaction Catalysed by Chromium-based MIL-101 Supported Au Nanoparticles. Mol. Catal. 2021, 501, 111363. [Google Scholar] [CrossRef]
- Liu, J.; Cui, J.; Chen, L.; Chen, J.; Zheng, H.; Oyama, S.T. Advantages of Tandem versus Simultaneous Operation: The Case of Isomerization/Hydrogenation of Terpinolene Epoxideto Terpinen-4-ol usinga Ni/TiO2-SiO2 Bifunctional Catalyst. Chem. Eng. Sci. 2022, 259, 117828. [Google Scholar] [CrossRef]
- Borguet, Y.; Sauvage, X.; Zaragoza, G.; Demonceau, A.; Delaude, L. Tandem Catalysis of Ring-closing Metathesis/AtomTransfer Radical Reactions with Homobimetallic Ruthenium-Arene Complexes. Beilstein J. Org. Chem. 2010, 6, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, Y.; Shi, Y.; Liu, W.; Tian, Z.; Zhang, X.; Zheng, L.; Hong, S.; Wei, M. Single-atom Catalysts with Metal-acid Synergistic Effect Toward Hydrodeoxygenation Tandem Reactions. Chem. Catal. 2023, 3, 100483. [Google Scholar] [CrossRef]
- Cho, H.J.; Xu, B. Enabling Selective Tandem Reactions via Catalyst Architecture Engineering. Trends Chem. 2020, 2, 929–941. [Google Scholar] [CrossRef]
- Anderson, A.E.; Baddeley, C.J.; Wright, P.A. Tuning Pd-nanoparticle@MIL-101(Cr) Catalysts for Tandem Reductive Amination. Catal. Lett. 2018, 148, 154–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellissier, H. Recent Developments in Enantioselective Multicatalyzed Tandem Reactions. Adv. Synth. Catal. 2020, 362, 2289–2325. [Google Scholar] [CrossRef]
- Reen, G.K.; Kumar, A.; Sharma, P. Recent Advances on the Transition-metal-catalyzed Synthesis of Imidazopyridines: An Updated Coverage. Beilstein J. Org. Chem. 2019, 15, 1612–1704. [Google Scholar] [CrossRef] [Green Version]
- Climent, M.J.; Corma, A.; Iborra, S.; Sabater, M.J. Heterogeneous Catalysis for Tandem Reactions. ACS Catal. 2014, 4, 870–891. [Google Scholar] [CrossRef]
- She, W.; Wang, J.; Li, X.; Li, J.; Mao, G.; Li, W.; Li, G. Bimetallic CuZn-MOFs derived Cu-ZnO/C catalyst for reductive amination of nitroarenes with aromatic aldehydes tandem reaction. Appl. Surf. Sci. 2021, 569, 151033. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, H.; Sun, Y.; Liu, X.; Zhang, M.; Luo, Y.; Gao, J.; Xu, J. Selective Tandem Hydrogenation and Rearrangement of Furfural to Cyclopentanone over CuNi Bimetallic Catalyst in Water. Chin. J. Catal. 2021, 42, 2216–2224. [Google Scholar] [CrossRef]
- George, J.; Kim, H.Y.; Oh, K. Cooperative Pd/Cu Catalysis to Spiro[indoline-2,3′-pyrrolidin]-2′-ones: Tandem Benzylation of α-Isocyano Lactams, Amine Addition, and N-Arylation. Org. Lett. 2019, 21, 5747–5752. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, H.; Chen, S.; Sheng, L.; Zhang, Z.; Wu, W.; Fan, M.; Wang, L.; Yang, B. Atomic Pd dispersion in triangular Cu nanosheets with dominant (111) plane as a tandem catalyst for highly efficient and selective electrodehalogenation. Appl. Catal. B Environ. 2023, 328, 122480. [Google Scholar] [CrossRef]
- Dong, Y.; Li, W.-H.; Dong, Y.-B. Dual-Metal N-Heterocyclic Carbene Complex (M= Au and Pd)-Functionalized UiO-67MOF for Alkyne Hydration-Suzuki Coupling Tandem Reaction. J. Org. Chem. 2021, 86, 1818–1826. [Google Scholar] [CrossRef]
- Nishad, R.C.; Kumar, S.; Rit, A. Hetero- and Homobimetallic Complexes Bridged by a Bis(NHC) Ligand: Synthesis via Selective Sequential Metalation and Catalytic Applications in Tandem Organic Transformations. Organometallics 2021, 40, 915–926. [Google Scholar] [CrossRef]
- Dehury, N.; Tripathy, S.K.; Sahoo, A.; Maity, N.; Patra, S. Facile Tandem Suzuki Coupling/Transfer Hydrogenation Reaction with a bis-Heteroscorpionate Pd–Ru Complex. Dalton Trans. 2014, 43, 16597–16600. [Google Scholar] [CrossRef] [PubMed]
- Pezük, L.G.; Șen, B.; Hahn, F.E.; Türkmen, H. Heterobimetallic Complexes Bridged by Imidazol{[4,5-f][1,10]-phenanthrolin}-2-ylidene: Synthesis and Catalytic Activity in Tandem Reactions. Organometallics 2019, 38, 593–601. [Google Scholar] [CrossRef]
- Mandegani, Z.; Nahaei, A.; Nikravesh, M.; Nabavizadeh, S.M.; Shahsavari, H.R.; Abu-Omar, M.M. Synthesis and Characterization of RhIII−MII (M = Pt, Pd) Heterobimetallic Complexes Based on a Bisphosphine Ligand: Tandem Reactions Using Ethanol. Organometallics 2020, 39, 3879–3891. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Wang, Y.; Yang, W.; Yu, Y. Nitrogen-richg-C3N4@AgPd Mott-Schottky heterojunction boosts photocatalytic hydrogen production from water and tandem reduction of NO3− and NO2−. J. Colloid Interface Sci. 2021, 581, 619–626. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, L.; Cao, J.; Jiang, W.; Xie, J.-X.; Zhu, C.; Wang, S.-Y.; Wei, Y.-L.; Zhao, X.-Y.; Bai, H.-C. Water-involved tandem conversion of arylethers to alcohols over metal phosphide catalyst. Chem. Eng. J. 2022, 435, 134911. [Google Scholar] [CrossRef]
- Jia, H.L.; Yang, Y.Y.; Chow, T.H.; Zhang, H.; Liu, X.Y.; Wang, J.F.; Zhang, C.-Y. Symmetry-Broken Au-Cu Heterostructures and their Tandem Catalysis Processin Electrochemical CO2 Reduction. Adv. Funct. Mater. 2021, 31, 2101255. [Google Scholar] [CrossRef]
- Li, X.-Q.; Duan, G.-Y.; Wang, R.; Han, L.-J.; Wang, Y.-F.; Xu, B.-H. Poly(ionicliquid)-based Bimetallic Tandem Catalysts for Highly Efficient Carbon Dioxide Electroreduction. Appl. Catal. B Environ. 2022, 313, 121459. [Google Scholar] [CrossRef]
- Balanta, A.; Godard, C.; Claver, C. Pd Nanoparticles for C–C Coupling Reactions. Chem. Soc. Rev. 2011, 40, 4973–4985. [Google Scholar] [CrossRef] [PubMed]
- Biffis, A.; Centomo, P.; DelZotto, A.; Zecca, M. Pd Metal Catalysts for Cross-Couplings and Related Reactions in the 21st Century: A Critical Review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef] [PubMed]
- Dobrounig, P.; Trobe, M.; Breinbauer, R. Sequential and Iterative Pd-catalyzed Cross-coupling Reactions in Organic Synthesis. Monatsh. Chem. 2017, 148, 3–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, M.; Miyoshi, N.; Hanada, K.; Kobayashi, S. Three-Component, One-Pot Tandem Sonogashira/Suzuki-Miyaura Coupling Reactions for the Synthesis of a Library of Ceramide-Transport Protein Inhibitors Designed In Silico. Asian J. Org. Chem. 2020, 9, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Wang, H.; Cao, Z.-Y.; Zhu, J.-W.; Liang, R.-X.; Hong, X.; Jia, Y.-X. Dearomative 1,4-Difunctionalization of Naphthalenes via Palladium-Catalyzed Tandem Heck/Suzuki Coupling Reaction. Nat. Commun. 2020, 11, 4380. [Google Scholar] [CrossRef]
- Matsude, A.; Hirano, K.; Miura, M. Highly Stereoselective Synthesis of 1,2-Disubstituted Indanes by Pd-Catalyzed Heck/Suzuki Sequence of Diarylmethyl Carbonates. Org. Lett. 2020, 22, 3190–3194. [Google Scholar] [CrossRef]
- Pagliaro, M.; Pandarus, V.; Ciriminna, R.; Béland, F.; Carà, P.D. Heterogeneous versus Homogeneous Palladium Catalysts for Cross-Coupling Reactions. ChemCatChem 2012, 4, 432–445. [Google Scholar] [CrossRef]
- Eremin, D.B.; Ananikov, V.P. Understanding Active Species in Catalytic Transformations: From Molecular Catalysis to Nanoparticles, Leaching, “Cocktails” of Catalysts and Dynamic Systems. Coord. Chem. Rev. 2017, 346, 2–19. [Google Scholar] [CrossRef]
- Lamb, C.J.C.; Nderitu, B.G.; McMurdo, G.; Tobin, J.M.; Vilela, F.; Lee, A.-L. Auto-Tandem Catalysis: PdII-Catalysed Dehydrogenation/Oxidative Heck Reaction of Cyclopentane-1,3-diones. Chem. Eur. J. 2017, 23, 18282–18288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Li, P.; Fu, H.; Dai, Q.; Hu, C. Palladium-Catalyzed Synthesis of Indolines from Aroyloxycarbamates through a Tandem Decarboxylative Amination/Heck/Annulation Reaction. Adv. Synth. Catal. 2019, 361, 192–200. [Google Scholar] [CrossRef]
- Song, J.; Chi, X.; Meng, L.; Zhao, P.; Sun, F.; Zhang, D.; Jiao, L.; Liu, Q.; Dong, Y.; Liu, H. Pd-Catalyzed Tandem Coupling Reaction of 2-gem-Dibromovinylanilines and N-Tosylhydrazones to Construct 2-(1-phenylvinyl)-indoles. Adv. Synth. Catal. 2019, 361, 3599–3604. [Google Scholar] [CrossRef]
- Revathi, L.; Ravindar, L.; Balakrishna, M.; Qin, H.-L. SO2F2 mediated dehydrative cross-coupling of alcohols with electron-deficient olefins in DMSO using Pd-catalyst: One-pot transformation of alcohols to 1,3-diene. Org. Chem. Front. 2019, 6, 796–800. [Google Scholar] [CrossRef]
- Manikandan, T.S.; Ramesh, R.; Semeril, D. The Tandem C–H/N–H Activation of N-Methyl Arylamide Catalyzed by Dinuclear Pd(II)Benzhydrazone Complex: A Concise Access to Phenanthridinone. Organometallics 2019, 38, 319–328. [Google Scholar] [CrossRef]
- Yadav, S.; Dash, C. One-pot Tandem Heck Alkynylation/cyclization Reactions Catalyzed by Bis(Pyrrolyl)pyridine Based Palladium Pincer Complexes. Tetrahedron 2020, 76, 131350. [Google Scholar] [CrossRef]
- Karu, R.; Gedu, S. Microwave Assisted Domino Heck Cyclization and Alkynylation: Synthesis of Alkyne Substituted Dihydrobenzofurans. Green Chem. 2018, 20, 369–374. [Google Scholar] [CrossRef]
- Ho, H.E.; Stephens, T.C.; Payne, T.J.; O’Brien, P.; Taylor, R.J.K.; Unsworth, W.P. Merging π-Acid and Pd Catalysis: Dearomatizing Spirocyclization/Cross-Coupling Cascade Reactions of Alkyne-Tethered Aromatics. ACS Catal. 2019, 9, 504–510. [Google Scholar] [CrossRef]
- Wei, W.-X.; Li, Y.; Wen, Y.-T.; Li, M.; Li, X.-S.; Wang, C.-T.; Liu, H.-C.; Xia, Y.; Zhang, B.-S.; Jiao, R.-Q.; et al. Experimental and Computational Studies of Palladium-Catalyzed Spirocyclization via a Narasaka–Heck/C(sp3 or sp2)–H Activation Cascade Reaction. JACS 2021, 143, 7868–7875. [Google Scholar] [CrossRef]
- Khan, F.; Fatima, M.; Shirzaei, M.; Vo, Y.; Amarasiri, M.; Banwell, M.G.; Ma, C.; Ward, J.S.; Gardiner, M.G. Tandem Ullmann–Goldberg Cross-Coupling/Cyclopalladation-Reductive Elimination Reactions and Related Sequences Leading to Polyfunctionalized Benzofurans, Indoles, and Phthalanes. Org. Lett. 2019, 21, 6342–6346. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.-M.; Xu, B.; Wu, L.; Wu, Y.; Qian, Y.; Zhou, L.; Liu, Y. Enantioselective Dicarbofunctionalization of Unactivated Alkenes by Pd-Catalyzed Tandem Heck/Suzuki-Coupling Reaction. Angew. Chem. Int. Ed. 2019, 58, 14653–14659. [Google Scholar] [CrossRef] [PubMed]
- Yokoya, M.; Ishiguro, T.; Sakairi, Y.; Kimura, S.; Morita, Y.; Yamanaka, M. Simple Strategy for Benzo[de]chromene-7,8-dione Synthesis via Tandem Sonogashira Coupling and Intramolecular Cyclization Reactions. Asian J. Org. Chem. 2022, 11, e202200534. [Google Scholar] [CrossRef]
- Teng, K.-X.; Niu, L.-Y.; Li, J.; Jia, L.; Yang, Q.-Z. An Unexpected Coupling-Reduction Tandem Reaction for the Synthesis of Alkenyl-Substituted BODIPYs. Chem. Commun. 2019, 55, 13761–13764. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, X.; Wang, H.; Chen, C.; Sun, P.; Mo, B.; Peng, J. Palladium-Catalyzed Tandem One-pot Synthesis of π-Expanded Imidazoles through a Sequential Heck and Oxidative Amination Reaction. Org. Biomol. Chem. 2019, 17, 4014–4023. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.B.; Choury, M.; Wagner, P.; Gulea, M. Tandem Double-Cross-Coupling/Hydrothiolation Reaction of 2-Sulfenyl Benzimidazoles with Boronic Acids. Org. Lett. 2019, 21, 5943–5947. [Google Scholar] [CrossRef]
- Luo, J.; Chen, G.-S.; Chen, S.-J.; Li, Z.-D.; Zhao, Y.-L.; Liu, Y.-L. One-Pot Tandem Protocol for the Synthesis of 1,3-Bis(β-aminoacrylate)-Substituted 2-Mercaptoimidazole Scaffolds. Adv. Synth. Catal. 2020, 362, 3635–3643. [Google Scholar] [CrossRef]
- Choi, H.; Shirley, H.J.; Aitken, H.R.M.; Schulte, T.; Söhnel, T.; Hume, P.A.; Brimble, M.A.; Furkert, D.P. Intermolecular Diels–Alder Cycloaddition/Cross-Coupling Sequences of 2-Bromo-1,3-butadienes. Org. Lett. 2020, 22, 1022–1027. [Google Scholar] [CrossRef]
- Arroniz, C.; Chaubet, G.; Anderson, E.A. Dual Oxidation State Tandem Catalysis in the Palladium-Catalyzed Isomerization of Alkynyl Epoxides to Furans. ACS Catal. 2018, 8, 8290–8295. [Google Scholar] [CrossRef]
- Yu, S.; Dai, L.; Shao, Y.; Li, R.; Chen, Z.; Lv, N.; Chen, J. Palladium-Catalyzed Tandem Reaction of Epoxynitriles with Arylboronic Acids in Aqueous Medium: Divergent Synthesis of Furans and Pyrroles. J. Org. Chem. Front. 2020, 7, 3439–3445. [Google Scholar] [CrossRef]
- Kathe, P.M.; Fleischer, I. Palladium-Catalyzed Tandem Isomerization/Hydrothiolation of Allylarenes. Org. Lett. 2019, 21, 2213–2217. [Google Scholar] [CrossRef]
- Han, H.; Yang, S.-D.; Xia, J.-B. Pd/Cu Cocatalyzed Oxidative Tandem C–H Aminocarbonylation and Dehydrogenation of Tryptamines: Synthesis of Carbolinones. J. Org. Chem. 2019, 84, 3357–3369. [Google Scholar] [CrossRef] [PubMed]
- Čarný, T.; Markovič, M.; Gracza, T.; Koóš, P. One-Step Synthesis of Isoindolo[2,1-a]indol-6-ones via Tandem Pd-Catalyzed Aminocarbonylation and C–HActivation. J. Org. Chem. 2019, 84, 12499–12507. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Y.; Zeng, Y.; Fan, Y.; Lin, A.; Yao, H. Palladium-Catalyzed Asymmetric Dearomative Carbonylation of Indoles. Org. Lett. 2022, 24, 3033–3037. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.; Zang, J.; Liu, M.; Zhang, S.; Jiang, G.; Ji, F. Palladium-Catalyzed Multistep Tandem Carbonylation/N-Dealkylation/Carbonylation Reaction: Access to Isatoic Anhydrides. J. Org. Chem. 2020, 85, 2672–2679. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.-C.; Zhuang, Y.-Y.; Jing, T.-H.; Shi, G.-H.; Guo, L.; Zhao, X.-L.; Lu, Y.; Liu, Y. Pd-Catalyzed Tandem Bis-Hydroaminocarbonylation of Terminal Alkynes for Synthesis of N-Aryl Substituted Succinimides with Involvement of Ionic P,O-hybrid Ligand. J. Catal. 2023, 417, 248–259. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Huang, H. Palladium-Catalyzed Tandem Carbonylative Diels-Alder Reaction for Construction of Bridged Polycyclic Skeletons. Org. Lett. 2021, 23, 2125–2129. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-J.; Zhao, L.-P.; Wang, L.; Tang, Y. Cyclization with Alkyl Substituted Methylene Malonate Enabling Concise Total Synthesis of Four Malagasy Alkaloids. CCS Chem. 2023, 5, 124–132. [Google Scholar] [CrossRef]
- Gu, Z.-Y.; Xia, J.-B. [3+1+1]Cyclization of vinyloxiranes with azides and CO by tandem palladium catalysis: Efficient synthesis of oxazolidinones. Org. Chem. Front. 2021, 8, 4112–4117. [Google Scholar] [CrossRef]
- Lokolkar, M.S.; Mane, P.A.; Dey, S.; Bhanage, B.M. Synthesis of 2-Substituted Indoles by Pd-Catalyzed Reductive Cyclization of 1-Halo-2-nitrobenzene with Alkynes. Eur. J. Org. Chem. 2022, 2022, e202101505. [Google Scholar] [CrossRef]
- Ding, L.; Niu, Y.-N.; Xia, X.-F. Pd-Catalyzed Tandem Isomerization/Cyclization for the Synthesis of Aromatic Oxazaheterocycles and Pyrido[3,4-b]indoles. J. Org. Chem. 2021, 86, 10032–10042. [Google Scholar] [CrossRef]
- Fillery, S.M.; Gregson, C.L.; Guérot, C.M. Expeditious Access to Functionalized Tricyclic Pyrrolo-Pyridones via Tandem or Sequential C–N/C–C Bond Formations. Org. Lett. 2019, 21, 9128–9132. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.J.; Joshi, G.; Yadav, U.P.; Maurya, A.K.; Agnihotri, V.K.; Kalra, S.; Kumar, R.; Singh, S.; Sawant, D.M. Exploration of Pd-Catalysed Four-Component Tandem Reaction for One-Pot Assembly of Pyrazolo[1,5-c]quinazolines as Potential EGFR Inhibitors. Bioorg. Chem. 2019, 93, 103314. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.J.; Joshi, G.; Sharma, P.; Maurya, A.K.; Metre, R.K.; Agnihotri, V.K.; Chandaluri, C.G.; Kumar, R.; Singh, S.; Sawant, D.M. Pd-Catalyzed Four-Component Sequential Reaction Delivers a Modular Fluorophore Platform for Cell Imaging. J. Org. Chem. 2019, 84, 3817–3825. [Google Scholar] [CrossRef]
- Gao, X.; Xia, M.; Yuan, C.; Zhou, L.; Sun, W.; Li, C.; Wu, B.; Zhu, D.; Zhang, C.; Zheng, B.; et al. EnantioselectiveSynthesis of Chiral Medium-Sized Cyclic Compounds via Tandem Cycloaddition/Cope Rearrangement Strategy. ACS Catal. 2019, 9, 1645–1654. [Google Scholar] [CrossRef]
- Ren, Z.-L.; Qiu, J.-Y.; Yuan, L.-L.; Yuan, Y.-F.; Cai, S.; Li, J.; Kong, C.; He, P.; Wang, L. Divergent Conversion of Double Isocyanides with Alkenyl Bromide to Polysubstituted Pyrroles and 4-Imino-4,5-dihydropyrrolo[3,4-b]pyrrol-6(1H)-one Derivatives by Pd-Catalyzed Tandem Cyclization Reactions. Org. Lett. 2022, 24, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tang, Z.; Zhang, C.; Wang, C.; Wu, L.; Qu, J.; Chen, Y. Pd-Catalyzed Regiodivergent Synthesis of Diverse Oxindoles Enabled by the Versatile Heck Reaction of Carbamoyl Chlorides. Org. Lett. 2020, 22, 3915–3921. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Wang, Z.; Chen, C.; Zhu, B.; Wang, Y.; Zhao, J.; Gong, J.; Han, M.; Liu, C. Palladium-Catalyzed Tandem Cyclization of Fluorinated Imidoyl Chlorides with 2-Bromophenylboronic Acid: Synthesis of 6-Fluoroalkyl-Phenanthridines. Tetrahedron 2019, 75, 1450–1456. [Google Scholar] [CrossRef]
- Dai, L.; Yu, S.; Xiong, W.; Chen, Z.; Xu, T.; Shao, Y.; Chen, J. Divergent Palladium-Catalyzed Tandem Reaction of Cyanomethyl Benzoates with Arylboronic Acids: Synthesis of Oxazoles and Isocoumarins. Adv. Synth. Catal. 2020, 362, 1893–1898. [Google Scholar] [CrossRef]
- Xu, T.; Shao, Y.; Dai, L.; Yu, S.; Cheng, T.; Chen, J. Pd-Catalyzed Tandem Reaction of 2-Aminostyryl Nitriles with Arylboronic Acids: Synthesis of 2-Arylquinolines. J. Org. Chem. 2019, 84, 13604–13614. [Google Scholar] [CrossRef]
- Yao, X.; Qi, L.; Li, R.; Zhen, Q.; Liu, J.; Zhao, Z.; Shao, Y.; Hu, M.; Chen, J. Palladium-Catalyzed Cascade Reactions of δ-Ketonitriles with Arylboronic Acids: Synthesis of Pyridines. ACS Comb. Sci. 2020, 22, 114–119. [Google Scholar] [CrossRef]
- Ye, X.; Xu, B.; Sun, J.; Dai, L.; Shao, Y.; Zhang, Y.; Chen, J. Pd-Catalyzed Approach for Assembling 9-Arylacridines via a Cascade Tandem Reaction of 2-(Arylamino)benzonitrile with Arylboronic Acidsin Water. J. Org. Chem. 2020, 85, 13004–13014. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Wang, J. Carbene-Catalyzed Tandem Isomerization/Cyclisation Strategy: Efficient Assembly of Benzoxazinones. Org. Chem. Front. 2019, 6, 1367–1371. [Google Scholar] [CrossRef]
- Patel, J.J.; Patel, A.P.; Chikhalia, K.H. An Efficient Pd Catalyzed Intramolecular Cyclization Reaction Followed by Formation of Benzimidazole Derivatives: Synthesis of Novel Quinolin-Fused Benzo[d] Azeto[1,2-a]benzimidazole Analogues. Synth. Commun. 2021, 51, 81–93. [Google Scholar] [CrossRef]
- Jin, T.; Suzuki, S.; Ho, H.E.; Matsuyama, H.; Kawata, M.; Terada, M. Pd-Catalyzed Indolization/peri-C–H Annulation/N-Dealkylation Cascade to Cyclopenta-Fused Acenaphtho[1,2-b]indole Scaffold. Org. Lett. 2021, 23, 9431–9435. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, S.; Cini, E.; Taddei, M.; Vinciarelli, G. Synthesis of 2-Substitued Indoles via Pd-Catalysed Cyclization in an Aqueous Micellar Medium. Molecules 2021, 26, 3917. [Google Scholar] [CrossRef]
- Xiong, W.; Chen, Z.; Shao, Y.; Li, R.; Hu, K.; Chen, J. The Synthesis of Fluorescent Benzofuro[2,3-c] Pyridines via Palladium-Catalyzed Heteroaromatic C–H Addition and Sequential Tandem Cyclization. Org. Chem. Front. 2020, 7, 756–762. [Google Scholar] [CrossRef]
- Błocka, A.; Chaładaj, W. Tandem Pd-Catalyzed Cyclization/Coupling of Non-Terminal Acetylenic Activated Methylenes with (Hetero)Aryl Bromides. Molecules 2022, 27, 630. [Google Scholar] [CrossRef]
- Hu, T.; Xu, K.; Ye, Z.; Zhu, K.; Wu, Y.; Zhang, F. Two-in-One Strategy for the Pd(II)-Catalyzed Tandem C–H Arylation/Decarboxylative Annulation Involved with Cyclic Diaryliodonium Salts. Org. Lett. 2019, 21, 7233–7237. [Google Scholar] [CrossRef]
- Ghosh, S.; Chattopadhyay, S.K. Unusual Regioselectivity in Palladium-Catalyzed Tandem C–H Arylation and C–H Amidation of cis-Cinnamyl Hydroxamates: Facile Synthesis of 3-Aryl-2-quinolones. Eur. J. Org. Chem. 2022, 2022, e202200391. [Google Scholar] [CrossRef]
- Domański, S.; Gatlik, B.; Chaładaj, W. Pd-Catalyzed Boroperfluoroalkylation of Alkynes Opens a Route to One-Pot Reductive Carboperfluoroalkylation of Alkynes with Perfluoroalkyl and Aryl Iodides. Org. Lett. 2019, 21, 5021–5025. [Google Scholar] [CrossRef]
- Fernández, N.P.; Gaube, G.; Woelk, K.J.; Burns, M.; Hruszkewycz, D.P.; Leitch, D.C. Palladium-Catalyzed Direct C–H Alkenylation with Enol Pivalates Proceeds via Reversible C–O Oxidative Addition to Pd(0). ACS Catal. 2022, 12, 6997–7003. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Shao, Y.; Zhang, F.; Chen, Z.; Lv, N.; Chen, J.; Li, R. Palladium(ii)-catalyzed three-component tandem reactions: Synthesis of multiplysubstituted quinolines. Org. Chem. Front. 2021, 8, 254–259. [Google Scholar] [CrossRef]
- Li, H.; Li, T.; Hsueh, Y.J.; Wu, X.; Xu, F.; Zhang, Y.J. Tandem arylation and regioselective allylic etherification of 2,3-allenol via Pd/B cooperative catalysis. Org. Biomol. Chem. 2019, 17, 8075–8078. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.-M.; Liu, M.; Wu, H.; Gou, T.; Hu, X.; Wang, B.-Q.; Hu, P.; Song, F.; Huang, G. Pd-Catalyzed tandem C–C/C–O/C–H single bond cleavage of 3-allyloxybenzocyclobutenols. Org. Chem. Front. 2021, 8, 3867–3875. [Google Scholar] [CrossRef]
- Li, W.; Zhang, C.; Lu, H.; Wang, Y.; Deng, G.; Liang, Y.; Yang, Y. Pd-Catalyzed One-Pot Synthesis of Vinylsilanes via a Three-Component Tandem Reaction. Org. Chem. Front. 2020, 7, 2075–2081. [Google Scholar] [CrossRef]
- Mahesha, C.K.; Borade, S.A.; Tank, D.; Bajaj, K.; Bhambri, H.; Mandal, S.K.; Sakhuja, R. Tandem Transformation of Indazolones to Quinazolinones through Pd-Catalyzed Carbene Insertionin to an N−N Bond. J. Org. Chem. 2023, 88, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Azizollahi, H.; Mehta, V.P.; García-López, J.-A. Pd-catalyzed cascade reactions involving skipped dienes: From double carbopalladation to remote C–C cleavage. Chem.Commun. 2019, 55, 10281–10284. [Google Scholar] [CrossRef]
- Giofrè, S.; Keller, M.; LoPresti, L.; Beccalli, E.M.; Molteni, L. Switchable Oxidative Reactions of N-allyl-2-Aminophenols: Palladium-Catalyzed Alkoxyacyloxylation vs an Intramolecular Diels–Alder Reaction. Org. Lett. 2021, 23, 7698–7702. [Google Scholar] [CrossRef]
- Xiong, Q.; Lu, J.; Shi, L.; Ran, G.-Y. Pd-Catalyzed Tandem [5+2] Cycloaddition/Ring Contraction of Phthalide-Derived Alkenes and Vinylethylene Carbonates for the Construction of Benzo-[5,5]-spiroketal Lactones. Org. Lett. 2022, 24, 3363–3367. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, T.-X.; Ma, J.; Zhang, G. Palladium-Catalyzed Three-Component Tandem Coupling–Carboannulation Reaction Leading to Polysubstituted [60]Fullerene-Fused Cyclopentanes. Org. Lett. 2020, 22, 284–289. [Google Scholar] [CrossRef]
- Zhang, M.; Weng, Z. Palladium-Catalyzed Tandem Synthesis of 2-Trifluoromethylthio(seleno)-Substituted Benzofused Heterocycles. Org. Lett. 2019, 21, 5838–5842. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-C.; Chen, X.-B.; Song, K.-L.; Wu, B.; Gan, W.-E.; Zheng, Z.-J.; Cao, J.; Xu, L.-W. Pd-Catalyzed Enantioselective Tandem C–C Bond Activation/Cacchi Reaction between Cyclobutanones and o-Ethynylanilines. Org. Lett. 2021, 23, 1309–1314. [Google Scholar] [CrossRef]
- Corma, A.; Navas, J.; Sabater, M.J. Advances in One-Pot Synthesis through Borrowing Hydrogen Catalysis. Chem. Rev. 2018, 118, 1410–1459. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Lin, D.; Xu, Z.; Li, Y. Pd/Cu bimetallic catalyst immobilized on PEI capped cellulose-polyamidoamine dendrimer: Synthesis, characterization, and application in Sonogashira reactions for the synthesis of alkynes and benzofurans. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129206. [Google Scholar] [CrossRef]
- Esfandiary, N.; Bagheri, S.; Heydari, A. Magnetic γ-Fe2O3@Cu-LDH intercalated with Palladium Cysteine: An efficient dual nanocatalyst in tandem C–N coupling and cyclization progress of synthesis quinolines. Appl. Clay Sci. 2020, 198, 105841. [Google Scholar] [CrossRef]
- Meng, J.; Chang, F.; Su, Y.; Liu, R.; Cheng, T.; Liu, G. Switchable Catalysts Used to Control Suzuki Cross-Coupling and Aza–Michael Addition/Asymmetric Transfer Hydrogenation Cascade Reactions. ACS Catal. 2019, 9, 8693–8701. [Google Scholar] [CrossRef]
- Rajabi, F.; Chia, C.H.; Sillanpää, M.; Voskressensky, L.G.; Luque, R. Cytosine Palladium Complex Supported on Ordered Mesoporous Silica as Highly Efficient and Reusable Nanocatalyst for One-Pot Oxidative Esterification of Aldehydes. Catalysts 2021, 11, 1482. [Google Scholar] [CrossRef]
- Sharma, A.K.; Bhattacherjee, D.; Sharma, N.; Giri, K.; Das, P. Supported-Pd catalyzed tandem approach for N-arylbenzamides synthesis. Mol. Catal. 2021, 516, 111948. [Google Scholar] [CrossRef]
- Amoo, C.C.; Xing, C.; Tsubaki, N.; Sun, J. Tandem Reactions over Zeolite-Based Catalysts in Syngas Conversion. ACS Cent. Sci. 2022, 8, 1047–1062. [Google Scholar] [CrossRef]
- Martínez-Edo, G.; Balmori, A.; Pontón, I.; MartídelRio, A.; Sánchez-García, D. Functionalized Ordered Mesoporous Silicas (MCM-41): Synthesis and Applications in Catalysis. Catalysts 2018, 8, 617. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, V.; Sharma, S. An overview of ordered mesoporous material SBA-15: Synthesis, functionalization and application in oxidation reactions. J. Porous Mater. 2017, 24, 741–749. [Google Scholar] [CrossRef]
- Yu, X.; Williams, C.T. Recent advances in the applications of mesoporous silica in heterogeneous catalysis. Catal. Sci. Technol. 2022, 12, 5765–5794. [Google Scholar] [CrossRef]
- Davidson, M.; Ji, Y.; Leong, G.J.; Kovach, N.C.; Trewyn, B.G.; Richards, R.M. Hybrid Mesoporous Silica/Noble-Metal Nanoparticle Materials – Synthesis and Catalytic Applications. ACS Appl. Nano Mater. 2018, 1, 4386–4400. [Google Scholar] [CrossRef]
- Singh, B.; Na, J.; Konarova, M.; Wakihara, T.; Yamauchi, Y.; Salomon, C.; Gawande, M.B. Functional Mesoporous Silica Nanomaterials for Catalysis and Environmental Applications. Bull. Chem. Soc. Jpn. 2020, 93, 1459–1496. [Google Scholar] [CrossRef]
- Hernández-Soto, M.C.; Erigoni, A.; Segarra, C.; Rey, F.; Díaz, U.; Gianotti, E.; Miletto, I.; Pera-Titus, M. Bifunctional hybrid organosiliceous catalysts for aldol condensation–hydrogenation tandem reactions of furfural in continuous-flow reactor. Appl. Catal. A Gen. 2022, 643, 118710. [Google Scholar] [CrossRef]
- Maties, G.; Gonzalez-Arellano, C.; Luque, R.; Montejano-Nares, E.; Ivars-Barceló, F.; Pineda, A. Trans-ferulic acid valorization into stilbene derivatives via tandem decarboxylation/Heck coupling using Pd/Al-SBA-15 materials. Mater. Today Chem. 2022, 25, 100971. [Google Scholar] [CrossRef]
- Scheithauer, M.; Grasselli, R.K.; Knözinger, H. Genesis and Structure of WOx/ZrO2 Solid Acid Catalysts. Langmuir 1998, 14, 3019–3029. [Google Scholar] [CrossRef]
- Zhou, W.; Luo, J.; Wang, Y.; Liu, J.; Zhao, Y.; Wang, S.; Ma, X. WOx domainsize, acid properties and mechanistic aspects of glycerol hydrogenolysis over Pt/WOx/ZrO2. Appl. Catal. B Environ. 2019, 242, 410–421. [Google Scholar] [CrossRef]
- Rodriguez-Gattorno, G.; Galano, A.; Torres-García, E. Surface acid–basic properties of WOx–ZrO2 and catalytic efficiency in oxidative desulfurization. Appl. Catal. B Environ. 2009, 92, 1–8. [Google Scholar] [CrossRef]
- Lwin, S.; Li, Y.; Frenkel, A.I.; Wachs, I.E. Nature of WOx Sites on SiO2 and Their Molecular Structure–Reactivity/Selectivity Relationships for Propylene Metathesis. ACS Catal. 2016, 6, 3061–3071. [Google Scholar] [CrossRef]
- Gayapan, K.; Sripinun, S.; Panpranot, J.; Praserthdam, P.; Assabumrungrat, S. Effect of pretreatment atmosphere of WOx/SiO2 catalysts on metathesis of ethylene and 2-butene to propylene. RSC Adv. 2018, 8, 11693–11704. [Google Scholar] [CrossRef] [PubMed]
- Watmanee, S.; Suriye, K.; Praserthdam, P.; Panpranot, J. Formation of isolated tungstate sites on hierarchical structured SiO2− and HY zeolite-supported WOx catalysts for propene metathesis. J. Catal. 2019, 376, 150–160. [Google Scholar] [CrossRef]
- Janampelli, S.; Sethia, G.; Darbha, S. Selective, bifunctional Cu–WOx/Al2O3 catalyst for hydrodeoxygenation of fatty acids. Catal. Sci. Technol. 2020, 10, 268–277. [Google Scholar] [CrossRef]
- García-Fernández, S.; Gandarias, I.; Requies, J.; Güemez, M.B.; Bennici, S.; Auroux, A.; Arias, P.L. New approaches to the Pt/WOx/Al2O3 catalytic system behavior for the selective glycerol hydrogenolysis to 1,3-propanediol. J. Catal. 2015, 323, 65–75. [Google Scholar] [CrossRef]
- Kim, H.; Numan, M.; Jo, C. Catalytic Dehydration of Ethanol over WOx Nanoparticles Supported on MFI (MobileFive) Zeolite Nanosheets. Catalysts 2019, 9, 670. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.; Luo, Z.; Xin, Y.; Jiang, C.; Gao, S.; Wang, Z.; Zhao, C. One-pot hydrogenolysis of cellulose to bioethanol over Pd-Cu-WOx/SiO2 catalysts. Fuel 2021, 292, 120311. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.-C.; Chang, G.-G.; Ke, S.-C.; Xia, T.; Hu, Z.-Y.; Yang, X.-Y. Synergistic catalysis of Pd nanoparticles withboth Lewis and Bronsted acid sites encapsulated within a sulfonated metal–organic frameworks toward one-pot tandem reactions. J. Colloid Interface Sci. 2019, 557, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Insyani, R.; Verma, D.; Cahyadi, H.S.; Kim, S.M.; Kim, S.K.; Karanwal, N.; Kim, J. One-pot di- and polysaccharides conversion to highly selective 2,5-dimethylfuran over Cu-Pd/Amino-functionalized Zr-based metal-organic framework (UiO-66(NH2))@SGO tandem catalyst. Appl. Catal. B Environ. 2019, 243, 337–354. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, K.; Liu, Y.; Luo, T.; Tian, G.; Li, J.; Zhang, S.; Chang, G.; Yang, X. A hierarchically multifunctional integrated catalyst with intimate and synergistic activesites for one-pot tandem catalysis. Inorg. Chem. Front. 2021, 8, 3463–3472. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Do, X.H.; Hwang, S.S.; Baek, K.-Y. Dual-functionalized ZIF-8 as an efficient acid-base bifunctional catalyst for the one-pot tandem reaction. Catal. Today 2021, 359, 124–132. [Google Scholar] [CrossRef]
- Fu, X.; Du, Y.; Liu, F.; Yang, J.; He, R.; Fu, G.; Yang, X. Double-shelled hollow polymer microspheres as acid and metallic colloid bi-functional catalyst for a deactalization-hydrogenation tandem reaction. Colloids Surf. A Physicochem. Eng. Asp. 2023, 659, 130833. [Google Scholar] [CrossRef]
- Hao, N.; Alper, K.; Patel, H.; Tekin, K.; Karagoz, S.; Ragauskas, A.J. One-stept ransformation of biomass to fuel precursors using a bi-functional combination of Pd/C and water tolerant Lewis acid. Fuel 2020, 277, 118200. [Google Scholar] [CrossRef]
- Raza, A.A.; Ravi, S.; Tajudeen, S.S.; Sheriff, A.K.I. Sulfonated covalent triazine polymer loaded with Pd nanoparticles as a bifunctional catalyst for one pot hydrogenation esterification reaction. J. Solid State Chem. 2021, 302, 122417. [Google Scholar] [CrossRef]
- Liu, J.; Wang, N.; Ma, L. Recent Advances in Covalent Organic Frameworks for Catalysis. Chem. Asian J. 2020, 15, 338–351. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, D. Covalent Organic Frameworks for Heterogeneous Catalysis: Principle, Current Status, and Challenges. ACS Cent. Sci. 2020, 6, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-Y.; Wang, T. Covalent Organic Frameworks in Catalytic Organic Synthesis. Adv. Synth. Catal. 2021, 363, 144–193. [Google Scholar] [CrossRef]
- Alsudairy, Z.; Brown, N.; Campbell, A.; Ambus, A.; Brown, B.; Smith-Petty, K.; Li, X. Covalent organic frameworks in heterogeneous catalysis: Recent advances and future perspective. Mater. Chem. Front. 2023, 7, 3298–3331. [Google Scholar] [CrossRef]
- Gong, K.; Li, C.; Zhang, D.; Lu, H.; Wang, Y.; Li, H.; Zhang, H. Sulfonic acid functionalized covalent organic frameworks as efficient catalyst for the one-pot tandem reactions. Mol. Catal. 2022, 519, 112139. [Google Scholar] [CrossRef]
- Wang, N.; Liu, J.; Li, X.; Ma, L. Selective control in the reductive amination of benzaldehyde towards corresponding amines over COF supported Pt, Pd, and Rh catalysts. Catal. Commun. 2023, 175, 106620. [Google Scholar] [CrossRef]
- Gonzalez-Granda, S.; Escot, L.; Lavandera, I.; Gotor-Fernandez, V. Chemoenzymatic Cascades Combining Biocatalysis and Transition Metal Catalysis for Asymmetric Synthesis. Angew. Chem. 2023, 62, e202217713. [Google Scholar] [CrossRef]
- Bering, L.; Thompson, J.; Micklefield, J. New reaction pathways by integrating chemo- and biocatalysis. Trends Chem. 2022, 4, 392–408. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Liu, P.; Kong, W.; Liu, J.; Jiang, Y. Integration of Chemo- and Bio-Catalysis to Intensify Bioprocesses. Phys. Sci. Rev. 2023. [Google Scholar] [CrossRef]
- Luan, P.; Liu, Y.; Li, Y.; Chen, R.; Huang, C.; Gao, J.; Hollmann, F.; Jiang, Y. Aqueous Chemoenzymatic One-Pot Enantioselective Synthesis of Tertiary α-Aryl Cycloketones via Pd-Catalyzed C–C Formation and Enzymatic C=C Asymmetric Hydrogenation. Green Chem. 2021, 23, 1960–1964. [Google Scholar] [CrossRef]
- Dawood, A.W.H.; Bassut, J.; de Souza, R.O.M.A.; Bornscheuer, U.T. Combination of the Suzuki–Miyaura Cross-Coupling Reaction with Engineered Transaminases. Chem. Eur. J. 2018, 24, 16009–16013. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.; Telzerow, A.; Ríos-Lombardía, N.; Steiner, K.; Schwab, H.; Morís, F.; Gröger, H.; González-Sabín, J. Enantioselective One-Pot Synthesis of Biaryl-Substituted Amines by Combining Palladium and Enzyme Catalysis in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 5486–5493. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, D.; Gotor, V.; Gotor-Fernandez, V. Stereoselective Synthesis of 1-Arylpropan-2-amines from Allylbenzenes through a Wacker-Tsuji Oxidation-Biotransamination Sequential Process. Adv. Synth. Catal. 2019, 361, 2582–2593. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, R.A.; Jha, A.K.; Kumar, P. Recent Advances in Wacker Oxidation: From Conventional to Modern Variants and Applications. Catal. Sci. Technol. 2020, 10, 7448–7470. [Google Scholar] [CrossRef]
- Albarran-Velo, J.; Gotor-Fernandez, V.; Lavandera, I. Markovnikov Wacker-Tsuji Oxidation of Allyl(hetero)arenes and Application in a One-Pot Photo-Metal-Biocatalytic Approach to Enantioenriched Amines and Alcohols. Adv. Synth. Catal. 2021, 363, 4096–4108. [Google Scholar] [CrossRef]
- Forero-Cortés, P.A.; Haydl, A.M. The 25th Anniversary of the Buchwald–Hartwig Amination: Development, Applications, and Outlook. Org. Process Res. Dev. 2019, 23, 1478–1483. [Google Scholar] [CrossRef]
- Cosgrove, S.C.; Thompson, M.P.; Ahmed, S.T.; Parmeggiani, F.; Turner, N.J. One-Pot Synthesis of Chiral N-Arylamines by Combining Biocatalytic Aminations with Buchwald–Hartwig N-Arylation. Angew. Chem. Int. Ed. 2020, 59, 18156–18160. [Google Scholar] [CrossRef]
- Heckmann, C.M.; Paradisi, F. GPhos Ligand Enables Production of Chiral N-Arylamines in a Telescoped Transaminase-Buchwald-Hartwig Amination Cascade in the Presence of Excess Amine Donor. Chem. Eur. J. 2021, 27, 16616–16620. [Google Scholar] [CrossRef] [PubMed]
- Coccia, F.; Tonucci, L.; DelBoccio, P.; Caporali, S.; Hollmann, F.; D’Alessandro, N. Stereoselective DoubleReduction of 3-Methyl-2-cyclohexenone, by Use of Palladium and Platinum Nanoparticles, in Tandem with Alcohol Dehydrogenase. Nanomaterials 2018, 8, 853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, R.A.; Zhu, X.; Ayepa, E.; You, S.; Wang, J. Advances in the One-Step Approach of Polymeric Materials Using Enzymatic Techniques. Polymers 2023, 15, 703. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, Y.; Xiong, J.; Li, J.; Xiao, H.; Li, X.; Gou, Q.; Ge, J. Enzyme-Metal-Single-Atom Hybrid Catalysts for One-Pot Chemoenzymatic Reactions. Chin. J. Catal. 2023, 44, 139–145. [Google Scholar] [CrossRef]
- Li, X.; Hu, X.; Qiao, Y.; Lu, T.; Bai, Y.; Xiong, J.; Li, X.; Gou, Q.; Ge, J. Enzyme-bimetallic hybrid catalyst for one-pot chemoenzymatic reactions. Chem. Eng. J. 2023, 452, 139356. [Google Scholar] [CrossRef]
- Collins, G.; Schmidt, M.; O’Dwyer, C.; Holmes, J.D.; McGlacken, G.P. The Origin of Shape Sensitivity in Palladium-Catalyzed Suzuki–Miyaura Cross Coupling Reactions. Angew. Chem. Int. Ed. 2014, 53, 4142–4145. [Google Scholar] [CrossRef] [PubMed]
- Deiana, L.; Rafi, A.A.; Naidu, V.R.; Tai, C.W.; Baeckvall, J.E.; Cordova, A. Artificial Plant Cell Walls as Multi-Catalyst Systems for Enzymatic Cooperative Asymmetric Catalysis in Non-Aqueous Media. Chem. Commun. 2021, 57, 8814–8817. [Google Scholar] [CrossRef]
- Craven, E.J.; Latham, J.; Shepherd, S.A.; Khan, I.; Diaz-Rodriguez, A.; Greaney, M.F.; Micklefield, J. Programmable Late-Stage C−H Bond Functionalization Enabled by Integration of Enzymes with Chemocatalysis. Nat Catal. 2021, 4, 385–394. [Google Scholar] [CrossRef]
- Huang, J.; Jiao, L.; Xu, W.; Fang, Q.; Wang, H.; Cai, X.; Yan, H.; Gu, W.; Zhu, C. Immobilizing Enzymes on Noble Metal Hydrogel Nanozymes with Synergistically Enhanced Peroxidase Activity for Ultrasensitive Immunoassays by Cascade Signal Amplification. ACS Appl. Mater. Interfaces 2021, 13, 33383–33391. [Google Scholar] [CrossRef]
- Ming, J.; Zhu, T.B.; Ye, Z.C.; Wang, J.J.; Chen, X.L.; Zheng, N.F. A Novel Cascade Nanoreactor Integrating Two-Dimensional Pd-Ru Nanozyme, Uricase and Red Blood Cell Membrane for Highly Efficient Hyperuricemia Treatment. Small 2021, 17, 2103645. [Google Scholar] [CrossRef]
- Zhang, N.; Hübner, R.; Wang, Y.; Zhang, E.; Zhou, Y.; Dong, S.; Wu, C. Surface-Functionalized Mesoporous Nanoparticles as Heterogeneous Supports to Transfer Bifunctional Catalysts into Organic Solvents for Tandem Catalysis. ACS Appl. Nano Mater. 2018, 1, 6378–6386. [Google Scholar] [CrossRef] [Green Version]
- Metzger, K.E.; Moyer, M.M.; Trewyn, B.G. Tandem Catalytic Systems Integrating Biocatalysts and Inorganic Catalysts Using Functionalized Porous Materials. ACS Catal. 2021, 11, 110–122. [Google Scholar] [CrossRef]
- Lackner, F.; Hiebler, K.; Grabner, B.; Gruber-Woelfler, H. Optimization of a Catalytic Chemoenzymatic Tandem Reaction for the Synthesis of Natural Stilbenes in Continuous Flow. Catalysts 2020, 10, 1404. [Google Scholar] [CrossRef]





































































Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikoshvili, L.Z.; Matveeva, V.G. Recent Progress in Pd-Catalyzed Tandem Processes. Catalysts 2023, 13, 1213. https://doi.org/10.3390/catal13081213
Nikoshvili LZ, Matveeva VG. Recent Progress in Pd-Catalyzed Tandem Processes. Catalysts. 2023; 13(8):1213. https://doi.org/10.3390/catal13081213
Chicago/Turabian StyleNikoshvili, Linda Z., and Valentina G. Matveeva. 2023. "Recent Progress in Pd-Catalyzed Tandem Processes" Catalysts 13, no. 8: 1213. https://doi.org/10.3390/catal13081213
APA StyleNikoshvili, L. Z., & Matveeva, V. G. (2023). Recent Progress in Pd-Catalyzed Tandem Processes. Catalysts, 13(8), 1213. https://doi.org/10.3390/catal13081213