Photocatalytic Degradation of 1,4-Dioxane by Heterostructured Bi2O3/Cu-MOF Composites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations
2.1.1. Morphology and Structure
2.1.2. XRD Analysis
2.1.3. XPS Analysis
2.1.4. BET and Particle Size Analysis
2.1.5. UV-Vis DRS Analysis
2.1.6. PL Spectra Analysis
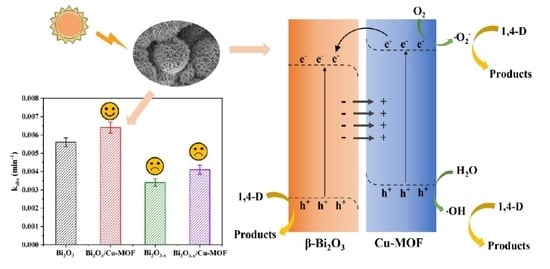
2.2. Photocatalytic Activity Performance
2.3. Photocatalytic Mechanisms
3. Methods
3.1. Materials
3.2. Synthesis of Composite Photocatalysts
3.2.1. Cu-MOF
3.2.2. Bi2O3 and Bi2O3/Cu-MOF
3.2.3. Bi2O3−x and Bi2O3−x/Cu-MOF
3.3. Characterizations
3.4. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coleman, H.M.; Vimonses, V.; Leslie, G.; Amal, R. Degradation of 1,4-dioxane in water using TiO2 based photocatalytic and H2O2/UV processes. J. Hazard. Mater. 2007, 146, 496–501. [Google Scholar] [CrossRef]
- Qiu, J.; Cheng, J.; Xie, Y.; Jiang, L.; Shi, P.; Li, X.; Swanda, R.V.; Zhou, J.; Wang, Y. 1,4-Dioxane exposure induces kidney damage in mice by perturbing specific renal metabolic pathways: An integrated omics insight into the underlying mechanisms. Chemosphere 2019, 228, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Chung, K.H.; Park, I.S.; Kim, S.C.; Kim, S.J.; Jung, S.C. Photocatalytic degradation of 1,4-dioxane using liquid phase plasma on visible light photocatalysts. J. Hazard. Mater. 2020, 399, 123087. [Google Scholar] [CrossRef]
- Wang, W.; Qiao, Z.; Lee, G.J.; Chen, H.; Ding, L.; Zhu, M.; Liu, N.; Wu, J.J. Preparation of ternary photocatalysts and their application in the degradation of 1,4-dioxane using O3/UV/photocatalyst process. Sep. Purif. Technol. 2020, 235, 116194. [Google Scholar] [CrossRef]
- Xu, X.; Liu, S.; Sun, P.; Guo, Z.; Smith, K.; Zhang, D.; Li, H.; Bedia, J.; Belver, C. Iron tungstate on nano-γ-alumina as photocatalyst for 1,4-dioxane solar degradation in water. J. Clean. Prod. 2022, 377, 134232. [Google Scholar] [CrossRef]
- Byrne, C.; Rhatigan, S.; Hermosilla, D.; Merayo, N.; Blanco, Á.; Michel, M.C.; Hinder, S.; Nolan, M.; Pillai, S.C. Modification of TiO2 with hBN: High temperature anatase phase stabilisation and photocatalytic degradation of 1,4-dioxane. J. Phys. Mater. 2020, 3, 015009. [Google Scholar] [CrossRef]
- Ali, N.S.; Kalash, K.R.; Ahmed, A.N.; Albayati, T.M. Performance of a solar photocatalysis reactor as pretreatment for wastewater via UV, UV/TiO2, and UV/H2O2 to control membrane fouling. Sci. Rep. 2022, 12, 16782. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, S.; Lu, Y.; Zhang, J.; Feng, Z.; Li, C. Controllable synthesis of α-Bi2O3 and γ-Bi2O3 with high photocatalytic activity by α-Bi2O3→γ-Bi2O3→α-Bi2O3 transformation in a facile precipitation method. J. Alloys Compd. 2016, 689, 787–799. [Google Scholar] [CrossRef]
- Han, S.; Li, J.; Yang, K.; Lin, J. Fabrication of a β-Bi2O3/BiOI heterojunction and its efficient photocatalysis for organic dye removal. Chin. J. Catal. 2015, 36, 2119–2126. [Google Scholar] [CrossRef]
- Ayekoe, P.Y.; Robert, D.; Goné, D.L. Preparation of effective TiO2/Bi2O3 photocatalysts for water treatment. Environ. Chem. Lett. 2016, 14, 387–393. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Jiang, X.; Chen, S.; Meng, S.; Fu, X. Design of a direct Z-scheme photocatalyst: Preparation and characterization of Bi2O3/g-C3N4 with high visible light activity. J. Hazard. Mater. 2014, 280, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, Y.; Zhang, Y.; Cao, J.J.; Li, H.; Bian, C.; Lee, S.C. Oxygen vacancy engineering of Bi2O3/Bi2O2CO3 heterojunctions: Implications of the interfacial charge transfer, NO adsorption and removal. Appl. Catal. B Environ. 2018, 231, 357–367. [Google Scholar] [CrossRef]
- Ke, J.; Liu, J.; Sun, H.; Zhang, H.; Duan, X.; Liang, P.; Li, X.; Tade, M.O.; Liu, S.; Wang, S. Facile assembly of Bi2O3/Bi2S3/MoS2 n-p heterojunction with layered n-Bi2O3 and p-MoS2 for enhanced photocatalytic water oxidation and pollutant degradation. Appl. Catal. B Environ. 2017, 200, 47–55. [Google Scholar] [CrossRef]
- Liu, J.; Ma, N.; Wu, W.; He, Q. Recent progress on photocatalytic heterostructures with full solar spectral responses. Chem. Eng. J. 2020, 393, 124719. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks, (1095-9203 (Electronic)). Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Akbarzadeh, E.; Soheili, H.Z.; Hosseinifard, M.; Gholami, M.R. Preparation and characterization of novel Ag3VO4/Cu-MOF/rGO heterojunction for photocatalytic degradation of organic pollutants. Mater. Res. Bull. 2020, 121, 110621. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, Z.; Feng, Y.; Lin, S.; Li, H.; Gao, X. Surface oxygen vacancy modified Bi2MoO6/MIL-88B(Fe) heterostructure with enhanced spatial charge separation at the bulk & interface. Appl. Catal. B Environ. 2020, 268, 118740. [Google Scholar]
- Ding, R.R.; Li, W.Q.; He, C.S.; Wang, Y.R.; Liu, X.C.; Zhou, G.N.; Mu, Y. Oxygen vacancy on hollow sphere CuFe2O4 as an efficient Fenton-like catalysis for organic pollutant degradation over a wide pH range. Appl. Catal. B Environ. 2021, 291, 120069. [Google Scholar] [CrossRef]
- Zou, X.; Mei, Z.; Jiang, J.; Guo, H. MOFs-derived Bi2O3@C with rich oxygen vacancies through rapid thermal annealing technology for photodegradation of tetracycline hydrochloride. Appl. Surf. Sci. 2022, 586, 152813. [Google Scholar] [CrossRef]
- Lin, K.S.; Adhikari, A.K.; Ku, C.-N.; Chiang, C.-L.; Kuo, H. Synthesis and characterization of porous HKUST-1 metal organic frameworks for hydrogen storage. Int. J. Hydrogen Energy 2012, 37, 13865–13871. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ji, M.; Wang, B.; Yin, S.; Xu, H.; Chen, Z.; Li, H. Carbon Quantum Dots Induced Ultrasmall BiOI Nanosheets with Assembled Hollow Structures for Broad Spectrum Photocatalytic Activity and Mechanism Insight. Langmuir 2016, 32, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.G.; Machado, F.L.A.; Junior, S.A.; Padrón-Hernández, E. Metal-organic framework: Structure and magnetic properties of [Cu3(BTC)2 (L)x·(CuO)y]n (L=H2O, DMF). J. Solid State Chem. 2017, 253, 1–5. [Google Scholar]
- Kumaraguru, S.; Nivetha, R.; Gopinath, K.; Sundaravadivel, E.; Almutairi, B.O.; Almutairi, M.H.; Mahboob, S.; Kavipriya, M.R.; Nicoletti, M.; Govindarajan, M. Synthesis of Cu-MOF/CeO2 nanocomposite and their evaluation of hydrogen production and cytotoxic activity. J. Mater. Res. Technol. 2022, 18, 1732–1745. [Google Scholar] [CrossRef]
- Lee, G.J.; Chien, Y.W.; Anandan, S.; Lv, C.; Dong, J.; Wu, J.J. Fabrication of metal-doped BiOI/MOF composite photocatalysts with enhanced photocatalytic performance. Int. J. Hydrogen Energy 2021, 46, 5949–5962. [Google Scholar] [CrossRef]
- Fan, M.; Wang, W.D.; Zhu, Y.; Sun, X.; Zhang, F.; Dong, Z. Palladium clusters confined in triazinyl-functionalized COFs with enhanced catalytic activity. Appl. Catal. B Environ. 2019, 257, 117942. [Google Scholar] [CrossRef]
- Hwang, H.; Shin, J.-H.; Lee, K.Y.; Choi, W. Facile one-pot transformation using structure-guided combustion waves of micro-nanostructured β-Bi2O3 to α-Bi2O3@C and analysis of electrochemical capacitance. Appl. Surf. Sci. 2018, 428, 422–431. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, S.; Cai, C.; Zhou, B. β- and α-Bi2O3 nanoparticles synthesized via microwave-assisted method and their photocatalytic activity towards the degradation of rhodamine B. Mater. Lett. 2011, 65, 988–990. [Google Scholar] [CrossRef]
- Zhou, H.; Wen, Z.; Liu, J.; Ke, J.; Duan, X.; Wang, S. Z-scheme plasmonic Ag decorated WO3/Bi2WO6 hybrids for enhanced photocatalytic abatement of chlorinated-VOCs under solar light irradiation. Appl. Catal. B Environ. 2019, 242, 76–84. [Google Scholar] [CrossRef]
- He, W.; Wei, Y.; Xiong, J.; Tang, Z.; Wang, Y.; Wang, X.; Deng, J.; Yu, X.; Zhang, X.; Zhao, Z. Boosting Selective Photocatalytic CO2 Reduction to CO over Dual-core@shell Structured Bi2O3/Bi2WO6@g-C3N4 Catalysts with Strong Interaction Interface. Sep. Purif. Technol. 2022, 300, 121850. [Google Scholar] [CrossRef]
- Kan, L.; Mu, W.; Chang, C.; Lian, F. Dual S-scheme graphitic carbon-doped α-Bi2O3/β-Bi2O3/Bi5O7I ternary heterojunction photocatalyst for the degradation of Bisphenol A. Sep. Purif. Technol. 2023, 312, 123388. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, J.; Li, J.; Qiu, K. Boosted photocatalytic removal of tetracycline on S-scheme Bi12O17Cl2/α-Bi2O3 heterojunctions with rich oxygen vacancies. Appl. Surf. Sci. 2021, 563, 150246. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, M.; Zhao, X.; Wang, H.; Huo, P. Fabricated local surface plasmon resonance Cu2O/Ni-MOF hierarchical heterostructure photocatalysts for enhanced photoreduction of CO2. J. Environ. Chem. Eng. 2023, 11, 109504. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, W.; Liu, N.; Huang, H.T.; Karuppasamy, L.; Yang, H.J.; Liu, C.H.; Wu, J.J. Synthesis of MOF/MoS2 composite photocatalysts with enhanced photocatalytic performance for hydrogen evolution from water splitting. Int. J. Hydrogen Energy 2022, 47, 40755–40767. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Bhardwaj, M.; Yadav, P.; Ogale, S. g-C3N4/NiAl-LDH 2D/2D Hybrid Heterojunction for High-Performance Photocatalytic Reduction of CO2 into Renewable Fuels. ACS Appl. Mater. Interfaces 2018, 10, 2667–2678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, M.; Wu, X.; Li, C.; Song, B.; Ma, X.; Liu, S.J.A.S.S. Insights into Boosting Photoelectrochemical Performance Over Cu3(BTC)2 Passivated Cu2O Nanorod Arrays. Adv. Sustain. Syst. 2022, 6, 2200272. [Google Scholar] [CrossRef]
- Wang, W.M.; Tseng, S.J.; Huang, Y.S.; Wu, Q.Y.; Wang, W.L.; Wu, J.J. Hollow-structured Pd/TiO2 as a dual functional photocatalyst for methyl orange oxidation and selective reduction of nitrate into nitrogen. J. Ind. Eng. Chem. 2023, 119, 386–394. [Google Scholar] [CrossRef]
- Wang, W.; Li, B.; Yang, H.J.; Liu, Y.; Gurusamy, L.; Karuppasamy, L.; Wu, J.J. Photocatalytic Hydrogen Evolution from Water Splitting Using Core-Shell Structured Cu/ZnS/COF Composites. Nanomaterials 2021, 11, 3380. [Google Scholar] [CrossRef]
- Wang, M.; Chen, J.; Hu, L.; Wei, Y.; Xu, Y.; Wang, C.; Gao, P.; Liu, Y.; Liu, C.; Song, Y.; et al. Heterogeneous interfacial photocatalysis for the inactivation of Karenia mikimotoi by Bi2O3 loaded onto a copper metal organic framework (Bi2O3@Cu-MOF) under visible light. Chem. Eng. J. 2023, 456, 141154. [Google Scholar] [CrossRef]
- Ren, Y.; Zeng, D.; Ong, W.-J. Interfacial engineering of graphitic carbon nitride (g-C3N4)-based metal sulfide heterojunction photocatalysts for energy conversion: A review. Chin. J. Catal. 2019, 40, 289–319. [Google Scholar] [CrossRef]
- Xu, X.; Liu, S.; Cui, Y.; Wang, X.; Smith, K.; Wang, Y. Solar-Driven Removal of 1,4-Dioxane Using WO3/nγ-Al2O3 Nano-catalyst in Water. Catalysts 2019, 9, 389. [Google Scholar] [CrossRef] [Green Version]
- Youn, N.K.; Heo, J.E.; Joo, O.S.; Lee, H.; Kim, J.; Min, B.K. The effect of dissolved oxygen on the 1,4-dioxane degradation with TiO2 and Au–TiO2 photocatalysts. J. Hazard. Mater. 2010, 177, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Min, B.K.; Heo, J.E.; Youn, N.K.; Joo, O.S.; Lee, H.; Kim, J.H.; Kim, H.S. Tuning of the photocatalytic 1,4-dioxane degradation with surface plasmon resonance of gold nanoparticles on titania. Catal. Commun. 2009, 10, 712–715. [Google Scholar] [CrossRef]
- Samy, M.; Alalm, M.G.; Khalil, M.N.; Ezeldean, E.; El-Dissouky, A.; Nasr, M.; Tawfik, A. Treatment of hazardous landfill leachate containing 1,4 dioxane by biochar-based photocatalysts in a solar photo-oxidation reactor. J. Environ. Manag. 2023, 332, 117402. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Y.; Zhao, J.; Song, Y.; Huang, Z.; Gao, F.; Li, N.; Li, Y. Induced Aqueous Synthesis of Metastable β-Bi2O3 Microcrystals for Visible-Light Photocatalyst Study. Cryst. Growth Des. 2015, 15, 1031–1042. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, R.; Lou, J.; Yuan, J.; Xu, J.; Fan, X. Novel marigold-like CuO@Cu-based MOFs composite photocatalyst for high-performance removal of alkylphenol ethoxylate under visible light. J. Environ. Chem. Eng. 2021, 9, 106434. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Elhag, S.A.O.; Adam, R.A.O.X.; Habibi-Yangjeh, A.; Liu, X.; Willander, M.; Nur, O.A.O.X. n-n ZnO-Ag2CrO4 heterojunction photoelectrodes with enhanced visible-light photoelectrochemical properties. RSC Adv. 2019, 9, 7992. [Google Scholar] [CrossRef]
- Ghosh, S.; Bera, S.; Singh, A.; Basu, S.; Basu, R.N. Hierarchical Bi2WO6/BiFeWO6 n-n heterojunction as an efficient photocatalyst for water splitting under visible light. J. Alloys Compd. 2022, 919, 165700. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A. Novel ZnO/Ag2CrO4 nanocomposites with n–n heterojunctions as excellent photocatalysts for degradation of different pollutants under visible light. J. Mater. Sci. Mater. Electron. 2016, 27, 4098–4108. [Google Scholar] [CrossRef]
- Gao, C.; Liu, G.; Liu, X.; Wang, X.; Liu, M.; Chen, Y.; Jiang, X.; Wang, G.; Teng, Z.; Yang, W. Flower-like n-Bi2O3/n-BiOCl heterojunction with excellent photocatalytic performance for visible light degradation of Bisphenol A and Methylene blue. J. Alloys Compd. 2022, 929, 167296. [Google Scholar] [CrossRef]










| Photocatalyst | Bi2O3 | Bi2O3/Cu-MOF | Bi2O3−x | Bi2O3−x/Cu-MOF |
|---|---|---|---|---|
| SBET (m2/g) | 5.09 | 9.52 | 6.08 | 7.81 |
| Photocatalyst | Band Gap (eV) |
|---|---|
| Cu-MOF | 3.59 |
| Bi2O3 | 3.54 |
| Bi2O3/Cu-MOF | 3.46 |
| Bi2O3−x | 3.30 |
| Bi2O3−x/Cu-MOF | 2.19 |
| Photocatalyst | Light Source | Catalyst Dosage (g/L) | 1,4-D Concentration (mg/L) | Time (min) | 1,4-D Removal Efficiency (%) | References |
|---|---|---|---|---|---|---|
| Fe/nAl | Solar | 0.3 | 50 | 180 | about 22 | [5] |
| WO3/nγ-Al2O3 | Solar | 0.3 | 50 | 180 | 56.67 | [41] |
| TiO2 | Xenon light (2 kW) | 0.5 | 500 | 180 | about 10 | [42] |
| Au–TiO2 | Xenon light (2 kW) | 0.5 | 500 | 240 | 59 | [43] |
| Cu-ZnO | Solar | 0.3 | 355 | 180 | 43.9 | [44] |
| Bi2O3/Cu-MOF | Xenon light (350 W) | 0.06 | 50 | 180 | 68.7 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-M.; Zhang, L.; Wang, W.-L.; Huang, J.-Y.; Wu, Q.-Y.; Wu, J.J. Photocatalytic Degradation of 1,4-Dioxane by Heterostructured Bi2O3/Cu-MOF Composites. Catalysts 2023, 13, 1211. https://doi.org/10.3390/catal13081211
Wang W-M, Zhang L, Wang W-L, Huang J-Y, Wu Q-Y, Wu JJ. Photocatalytic Degradation of 1,4-Dioxane by Heterostructured Bi2O3/Cu-MOF Composites. Catalysts. 2023; 13(8):1211. https://doi.org/10.3390/catal13081211
Chicago/Turabian StyleWang, Wen-Min, Lu Zhang, Wen-Long Wang, Jin-Yi Huang, Qian-Yuan Wu, and Jerry J. Wu. 2023. "Photocatalytic Degradation of 1,4-Dioxane by Heterostructured Bi2O3/Cu-MOF Composites" Catalysts 13, no. 8: 1211. https://doi.org/10.3390/catal13081211
APA StyleWang, W. -M., Zhang, L., Wang, W. -L., Huang, J. -Y., Wu, Q. -Y., & Wu, J. J. (2023). Photocatalytic Degradation of 1,4-Dioxane by Heterostructured Bi2O3/Cu-MOF Composites. Catalysts, 13(8), 1211. https://doi.org/10.3390/catal13081211