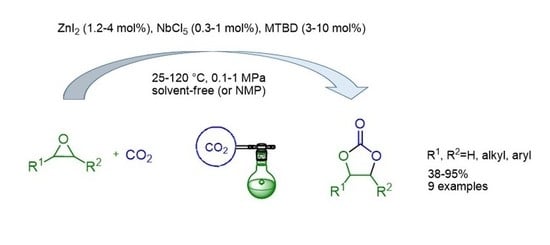
Zinc Iodide-Metal Chloride-Organic Base: An Efficient Catalytic System for Synthesis of Cyclic Carbonates from Carbon Dioxide and Epoxides under Ambient Conditions
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, B.-H.; Wang, J.-Q.; Sun, J.; Huang, Y.; Zhang, J.-P.; Zhang, X.-P.; Zhang, S.-J. Fixation of CO2 into cyclic carbonates catalyzed by ionic liquids: A multi-scale approach. Green Chem. 2015, 17, 108–122. [Google Scholar] [CrossRef]
- Fiorani, G.; Guo, W.; Kleij, A.W. Sustainable conversion of carbon dioxide: The advent of organocatalysis. Green Chem. 2015, 17, 1375–1389. [Google Scholar] [CrossRef]
- Comerford, J.W.; Ingram, I.D.V.; North, M.; Wu, X. Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings. Green Chem. 2015, 17, 1966–1987. [Google Scholar] [CrossRef]
- Motokura, K.; Itagaki, S.; Iwasawa, Y.; Miyaji, A.; Baba, T. Zinc-Accelerated Cycloaddition of Carbon Dioxide to Styrene Oxide Catalyzed by Pyrrolidinopyridinium Iodides. Top Catal. 2014, 57, 953–959. [Google Scholar] [CrossRef]
- Liu, M.; Liu, B.; Shi, L.; Wang, F.; Liang, L.; Sun, J. Melamine–ZnI2 as heterogeneous catalysts for efficient chemical fixation of carbon dioxide to cyclic carbonates. RSC Adv. 2015, 5, 960–966. [Google Scholar] [CrossRef]
- Liu, B.; Liu, M.; Liang, L.; Sun, J. Guanidine Hydrochloride/ZnI2 as Heterogeneous Catalyst for Conversion of CO2 and Epoxides to Cyclic Carbonates under Mild Conditions. Catalysts 2015, 5, 119–130. [Google Scholar] [CrossRef]
- Desens, W.; Kohrt, C.; Spannenberg, A.; Werner, T. A novel zinc based binary catalytic system for CO2 utilization under mild conditions. Org. Chem. Front. 2016, 3, 156–164. [Google Scholar] [CrossRef]
- Jasiak, K.; Siewniak, A.; Kopczyńska, K.; Chrobok, A.; Baj, S. Hydrogensulphate ionic liquids as an efficient catalyst for the synthesis of cyclic carbonates from carbon dioxide and epoxides. J. Chem. Technol. Biotechnol. 2016, 91, 2827–2833. [Google Scholar] [CrossRef]
- Xu, J.; Shang, J.-K.; Jiang, Q.; Wang, Y.; Li, Y.-X. Facile alkali-assisted synthesis of g-C3N4 materials and their high-performance catalytic application in solvent-free cycloaddition of CO2 to epoxides. RSC Adv. 2016, 6, 55382–55392. [Google Scholar] [CrossRef]
- Alaji, Z.; Safaei, E.; Wojtczak, A. Development of pyridine based o-aminophenolate zinc complexes as structurally tunable catalysts for CO2 fixation into cyclic carbonates. New J. Chem. 2017, 41, 10121–10131. [Google Scholar] [CrossRef]
- Xu, J.; Gan, Y.-L.; Hu, P.; Zheng, H.; Xue, B. Comprehensive insight into the support effect of graphitic carbon nitride for zinc halides on the catalytic transformation of CO2 into cyclic carbonates. Catal. Sci. Technol. 2018, 8, 5582–5593. [Google Scholar] [CrossRef]
- Ma, D.; Li, J.; Liu, K.; Li, B.; Li, C.; Shi, Z. Di-ionic multifunctional porous organic frameworks for efficient CO2 fixation under mild and co-catalyst free conditions. Green Chem. 2018, 20, 5285–5291. [Google Scholar] [CrossRef]
- Kim, D.; Ji, H.; Hur, M.Y.; Lee, W.; Kim, T.S.; Cho, D.-H. Polymer-Supported Zn-Containing Imidazolium Salt Ionic Liquids as Sustainable Catalysts for the Cycloaddition of CO2: A Kinetic Study and Response Surface Methodology. ACS Sustain. Chem. Eng. 2018, 6, 14743–14750. [Google Scholar] [CrossRef]
- Bondarenko, G.N.; Dvurechenskaya, E.G.; Ganina, O.G.; Alonso, F.; Beletskaya, I.P. Solvent-free synthesis of cyclic carbonates from CO2 and epoxides catalyzed by reusable alumina-supported zinc dichloride. Appl. Catal. B 2019, 254, 380–390. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Xu, P.; Yang, L.; Zhang, J.; Sun, J. Facile synthesis of zinc halide-based ionic liquid for efficient conversion of carbon dioxide to cyclic carbonates. Mol. Catal. 2020, 480, 110637. [Google Scholar] [CrossRef]
- Dong, J.-P.; Zhao, C.; Qiu, J.-J.; Liu, C.-M. Novel mono-and bi-functional phosphonium salts deriving from toxic phosphine off-gas as efficient catalysts for chemical fixation of CO2. J. Ind. Eng. Chem. 2020, 90, 95–104. [Google Scholar] [CrossRef]
- Sodpiban, O.; Phungpanya, C.; Gobbo, S.D.; Arayachukiat, S.; Piromchart, T.; D’Elia, V. Rational engineering of single-component heterogeneous catalysts based on abundant metal centers for the mild conversion of pure and impure CO2 to cyclic carbonates. Chem. Eng. J. 2021, 422, 129930. [Google Scholar] [CrossRef]
- D’Elia, V.; Kleij, A.W. Surface science approach to the heterogeneous cycloaddition of CO2 to epoxides catalyzed by site-isolated metal complexes and single atoms: A review. Green Chem. Eng. 2022, 3, 210–227. [Google Scholar] [CrossRef]
- Lan, D.-H.; Chen, K.; Zhu, H.-J.; Shen, J.; Wu, S.-S.; Au, C.-T.; Yi, B.; Yin, S.F. Hexamethylenetetramine Complexes as Easy-to-Handle and Efficient Lewis Acid-Base Heterogeneous Catalysts for Coupling of CO2 under Mild Conditions. Ind. Eng. Chem. Res. 2022, 61, 11390–11396. [Google Scholar] [CrossRef]
- Monassier, A.; D’Elia, V.; Cokoja, M.; Dong, H.; Pelletier, J.D.A.; Basset, J.-M.; Kühn, F.E. Synthesis of Cyclic Carbonates from Epoxides and CO2 under Mild Conditions Using a Simple, Highly Efficient Niobium-Based Catalyst. ChemCatChem 2013, 5, 1321–1324. [Google Scholar] [CrossRef]
- Wilhelm, M.E.; Anthofer, M.H.; Reich, R.M.; D’Elia, V.; Basset, J.-M.; Herrmann, W.A.; Cokoja, M.; Kühn, F.E. Niobium(V) chloride and imidazolium bromides as efficient dual catalyst systems for the cycloaddition of carbon dioxide and propylene oxide. Catal. Sci. Technol. 2014, 4, 1638–1643. [Google Scholar] [CrossRef]
- D’Elia, V.; Ghani, A.A.; Monassier, A.; Sofack-Kreutzer, J.; Pelletier, J.D.A.; Drees, M.; Vummaleti, S.V.C.; Poater, A.; Cavallo, L.; Cokoja, M.; et al. Dynamics of the NbCl5-Catalyzed Cycloaddition of Propylene Oxide and CO2: Assessing the Dual Role of the Nucleophilic Co-Catalysts. Chem. Eur. J. 2014, 20, 11870–11882. [Google Scholar] [CrossRef]
- D’Elia, V.; Dong, H.; Rossini, A.J.; Widdifield, C.M.; Vummaleti, S.V.C.; Minenkov, Y.; Poater, A.; Abou-Hamad, E.; Pelletier, J.D.A.; Cavallo, L.; et al. Cooperative Effect of Monopodal Silica-Supported Niobium Complex Pairs Enhancing Catalytic Cyclic Carbonate Production. J. Am. Chem. Soc. 2015, 137, 7728–7739. [Google Scholar]
- Wu, X.; Wang, M.; Xie, Y.; Chen, C.; Li, K.; Yuan, M.; Zhao, X.; Hou, Z. Carboxymethyl cellulose supported ionic liquid as a heterogeneous catalyst for the cycloaddition of CO2 to cyclic carbonate. Appl. Catal. A Gen. 2016, 519, 146–154. [Google Scholar] [CrossRef]
- Barbarini, A.; Maggi, R.; Mazzacani, A.; Mori, G.; Sartori, G.; Sartorio, R. Cycloaddition of CO2 to epoxides over both homogeneous and silica-supported guanidine catalysts. Tetrahedron Lett. 2003, 44, 2931–2934. [Google Scholar] [CrossRef]
- Kuznetsova, S.A.; Rulev, Y.A.; Larionov, V.A.; Smol’yakov, A.F.; Zubavichus, Y.V.; Maleev, V.I.; North, M.; Saghyan, A.S.; Belokon, Y.N. Self-Assembled Ionic Composites of Negatively Charged Zn(salen) Complexes and Triphenylmethane Derived Polycations as Recyclable Catalysts for the Addition of Carbon Dioxide to Epoxides. ChemCatChem 2019, 11, 511–519. [Google Scholar] [CrossRef]
- Janeta, M.; Lis, T.; Szafert, S. Zinc Imine Polyhedral Oligomeric Silsesquioxane as a Quattro-Site Catalyst for the Synthesis of Cyclic Carbonates from Epoxides and Low-Pressure CO2. Chem. Eur. J. 2020, 26, 13686–13697. [Google Scholar] [CrossRef]
- Bondarenko, G.N.; Ganina, O.G.; Lysova, A.A.; Fedin, V.P.; Beletskaya, I.P. Cyclic carbonates synthesis from epoxides and CO2 over NIIC-10 metal-organic frameworks. J. CO2 Util. 2021, 53, 101718. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Liu, Y.; Chen, Z.; Yang, H.; Yue, Z.; Fang, Q.; Zhi, Y.; Shan, S. Zn and N co-doped porous carbon nanosheets for photothermally-driven CO2 cycloaddition. J. Catal. 2022, 407, 65–76. [Google Scholar] [CrossRef]
- Saengsaen, S.; Gobbo, S.D.; D’Elia, V. Exploring the potential of nanosized oxides of zinc and tin as recyclable catalytic components for the synthesis of cyclic organic carbonates under atmospheric CO2 pressure. Chem. Eng. Res. Des. 2023, 191, 630–645. [Google Scholar] [CrossRef]
- Martín, C.; Fiorani, G.; Kleij, A.W. Recent Advances in the Catalytic Preparation of Cyclic Organic Carbonates. ACS Catal. 2015, 5, 1353–1370. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Lamb, K.J.; North, M. Cr(salophen) Complex Catalyzed Cyclic Carbonate Synthesis at Ambient Temperature And Pressure. ACS Catal. 2016, 6, 5012–5025. [Google Scholar] [CrossRef]
- Wu, X.; North, M. A Bimetallic Aluminium(Salphen) Complex for the Synthesis of Cyclic Carbonates from Epoxides and Carbon Dioxide. ChemSusChem 2017, 10, 74–78. [Google Scholar] [CrossRef]
- Steinbauer, J.; Spannenberg, A.; Werner, T. An in situ formed Ca2+–crown ether complex and its use in CO2-fixation reactions with terminal and internal epoxides. Green Chem. 2017, 19, 3769–3779. [Google Scholar] [CrossRef]
- Chowdhury, A.H.; Bhanja, P.; Salam, N.; Bhaumik, A.; Islam, S.M. Magnesium oxide as an efficient catalyst for CO2 fixation and N-formylation reactions under ambient conditions. Mol. Catal. 2018, 450, 46–54. [Google Scholar] [CrossRef]
- Shaikh, R.R.; Pornpraprom, S.; D’Elia, V. Catalytic Strategies for the Cycloaddition of Pure, Diluted, and Waste CO2 to Epoxides under Ambient Conditions. ACS Catal. 2018, 8, 419–450. [Google Scholar] [CrossRef]
- Bresciani, G.; Bortoluzzi, M.; Marchetti, F.; Pampaloni, G. Iron(III) N,N-Dialkylcarbamate-Catalyzed Formation of Cyclic Carbonates from CO2 and Epoxides under Ambient Conditions by Dynamic CO2 Trapping as Carbamato Ligands. ChemSusChem 2018, 11, 2737–2743. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hyun, K.; Ahn, D.; Kim, R.; Park, M.H.; Kim, Y. Efficient Aluminum Catalysts for the Chemical Conversion of CO2 into Cyclic Carbonates at Room Temperature and Atmospheric CO2 Pressure. ChemSusChem 2019, 12, 4211–4220. [Google Scholar] [CrossRef]
- Bayer, U.; Werner, D.; Maichle-Mössmer, C.; Anwander, R. Effective and Reversible Carbon Dioxide Insertion into Cerium Pyrazolates. Angew. Chem. Int. Ed. 2020, 59, 5830–5836. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Chowdhury, A.H.; Singha, P.; Banerjee, A.; Islam, S.M.; Bala, T. Morphology of ZnO triggered versatile catalytic reactions towards CO2 fixation and acylation of amines at optimized reaction conditions. Mol. Catal. 2020, 493, 111070. [Google Scholar] [CrossRef]
- Aomchad, V.; Gobbo, S.D.; Yingcharoen, P.; Poater, A.; D’Elia, V. Exploring the potential of group III salen complexes for the conversion of CO2 under ambient conditions. Catal. Today 2021, 375, 324–334. [Google Scholar] [CrossRef]
- Emelyanov, M.A.; Stoletova, N.V.; Lisov, A.A.; Medvedev, M.G.; Smol’yakov, A.F.; Maleev, V.I.; Larionov, V.A. An octahedral cobalt(III) complex based on cheap 1,2-phenylenediamine as a bifunctional metal-templated hydrogen bond donor catalyst for fixation of CO2 with epoxides under ambient conditions. Inorg. Chem. Front. 2021, 8, 3871–3884. [Google Scholar] [CrossRef]
- Gao, W.-Y.; Wojtas, L.; Ma, S. A porous metal–metalloporphyrin framework featuring high-density active sites for chemical fixation of CO2 under ambient conditions. Chem. Commun. 2014, 50, 5316–5318. [Google Scholar] [CrossRef]
- Dutta, G.; Jana, A.K.; Natarajan, S. Chemical Fixation of CO2 and Other Heterogeneous Catalytic Studies by Employing a Layered Cu-Porphyrin Prepared Through Single-Crystal to Single-Crystal Exchange of a Zn Analogue. Chem. Asian J. 2018, 13, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Zhang, Y.; Liu, L.; Han, Z.-B.; Yuan, D.-Q. Pentanuclear Yb(III) cluster-based metal-organic frameworks as heterogeneous catalysts for CO2 conversion. Appl. Catal. B Environ. 2017, 219, 603–610. [Google Scholar] [CrossRef]
- Wang, S.; Song, K.; Zhang, C.; Shu, Y.; Li, T.; Tan, B. A novel metalporphyrin-based microporous organic polymer with high CO2 uptake and efficient chemical conversion of CO2 under ambient conditions. J. Mater. Chem. A 2017, 5, 1509–1515. [Google Scholar] [CrossRef]
- Sun, Q.; Jin, Y.; Aguila, B.; Meng, X.; Ma, S.; Xiao, F.-S. Porous Ionic Polymers as a Robust and Efficient Platform for Capture and Chemical Fixation of Atmospheric CO2. ChemSusChem 2017, 10, 1160–1165. [Google Scholar] [CrossRef]
- Zakharova, M.V.; Kleitz, F.; Fontaine, F.-G. Carbon Dioxide Oversolubility in Nanoconfined Liquids for the Synthesis of Cyclic Carbonates. ChemCatChem 2017, 9, 1886–1890. [Google Scholar] [CrossRef]
- Khatun, R.; Bhanja, P.; Molla, R.A.; Ghosh, S.; Bhaumik, A.; Islam, S.M. Functionalized SBA-15 material with grafted CO2H group as an efficient heterogeneous acid catalyst for the fixation of CO2 on epoxides under atmospheric pressure. Mol. Catal. 2017, 434, 25–31. [Google Scholar] [CrossRef]
- Prajapati, P.K.; Kumar, A.; Jain, S.L. First Photocatalytic Synthesis of Cyclic Carbonates from CO2 and Epoxides Using CoPc/TiO2 Hybrid under Mild Conditions. ACS Sustain. Chem. Eng. 2018, 6, 7799–7809. [Google Scholar] [CrossRef]
- Biswas, S.; Khatun, R.; Sengupta, M.; Islam, S.M. Polystyrene supported Zinc complex as an efficient catalyst for cyclic carbonate formation via CO2 fixation under atmospheric pressure and organic carbamates production. Mol. Catal. 2018, 452, 129–137. [Google Scholar] [CrossRef]
- Mondal, R.K.; Riyajuddin, S.; Ghosh, A.; Ghosh, S.; Ghosh, K.; Islam, S.M. Polymer immobilized [Mg@PS-anthra] complex: An efficient recyclable heterogeneous catalyst for the incorporation of carbon dioxide into oxiranes at atmospheric pressure and Knoevenagel condensation reaction under solvent free condition. J. Organomet. Chem. 2019, 880, 322–332. [Google Scholar] [CrossRef]
- Aoyagi, N.; Furusho, Y.; Endo, T. Convenient synthesis of cyclic carbonates from CO2 and epoxides by simple secondary and primary ammonium iodides as metal-free catalysts under mild conditions and its application to synthesis of polymer bearing cyclic carbonate moiety. J. Poly. Sci. Part A Polym. Chem. 2013, 51, 1230–1242. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, G.-X.; Zhang, W.-Z.; Lu, X.-B. CO2 Adducts of Phosphorus Ylides: Highly Active Organocatalysts for Carbon Dioxide Transformation. ACS Catal. 2015, 5, 6773–6779. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Kodama, K.; Hirose, T. An efficient metal- and solvent-free organocatalytic system for chemical fixation of CO2 into cyclic carbonates under mild conditions. Green Chem. 2016, 18, 1229–1233. [Google Scholar] [CrossRef]
- Yuan, G.; Zhao, Y.; Wu, Y.; Li, R.; Chen, Y.; Xu, D.; Liu, Z. Cooperative effect from cation and anion of pyridine-containing anion-based ionic liquids for catalysing CO2 transformation at ambient conditions. Sci. China Chem. 2017, 60, 958–963. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Zhao, Y.; Kodama, K.; Hirose, T. Efficient and practical organocatalytic system for the synthesis of cyclic carbonates from carbon dioxide and epoxides: 3-hydroxypyridine/tetra-n-butylammonium iodide. Tetrahedron 2017, 73, 1190–1195. [Google Scholar] [CrossRef]
- Yang, X.; Zou, Q.; Zhao, T.; Chen, P.; Liu, Z.; Liu, F.; Lin, Q. Deep Eutectic Solvents as Efficient Catalysts for Fixation of CO2 to Cyclic Carbonates at Ambient Temperature and Pressure through Synergetic Catalysis. ACS Sustain. Chem. Eng. 2021, 9, 10437–10443. [Google Scholar] [CrossRef]
- Saini, S.; Khan, S.R.; Gour, N.K.; Deka, R.C.; Jain, S.L. Metal-free, redox-neutral, and visible light-triggered coupling of CO2 with epoxides to cyclic carbonates at atmospheric pressure. Green Chem. 2022, 24, 3644–3650. [Google Scholar] [CrossRef]
- Mihara, M.; Moroga, K.; Nakai, T.; Ito, T.; Ohno, T.; Mizuno, T. Selective Synthesis of Carbonates from Glycerol, CO2, and Alkyl Halides Using tert-Butyltetramethylguanidine. Synlett 2018, 29, 1759–1764. [Google Scholar] [CrossRef]
- Dutta, B.; Sofack-Kreutzer, J.; Ghani, A.A.; D’Elia, V.; Pelletier, J.D.A.; Cokoja, M.; Kühn, F.E.; Basset, J.-M. Nucleophile-directed selectivity towards linear carbonates in the niobium pentaethoxide-catalysed cycloaddition of CO2 and propylene oxide. Catal. Sci. Technol. 2014, 4, 1534–1538. [Google Scholar] [CrossRef]
- Arayachukiat, S.; Yingcharoen, P.; Vummaleti, S.V.C.; Cavallo, L.; Poater, A.; D’Elia, V. Cycloaddition of CO2 to challenging N-tosyl aziridines using a halogen-free niobium complex: Catalytic activity and mechanistic insights. Mol. Catal. 2017, 443, 280–285. [Google Scholar] [CrossRef]
- Natongchai, W.; Posada-Pérez, S.; Phungpanya, C.; Luque-Urrutia, J.A.; Solà, M.; D’Elia, V.; Poater, A. Enhancing the Catalytic Performance of Group I, II Metal Halides in the Cycloaddition of CO2 to Epoxides under Atmospheric Conditions by Cooperation with Homogeneous and Heterogeneous Highly Nucleophilic Aminopyridines: Experimental and Theoretical Study. J. Org. Chem. 2022, 87, 2873–2886. [Google Scholar] [CrossRef]
- Jing, H.; Nguyen, S.T. SnCl4-organic base: Highly efficient catalyst system for coupling reaction of CO2 and epoxides. J. Mol. Catal. A: Chem. 2007, 261, 12–15. [Google Scholar] [CrossRef]
- Zhu, J.; Usov, P.M.; Xu, W.; Celis-Salazar, P.J.; Lin, S.; Kessinger, M.C.; Landaverde-Alvarado, C.; Cai, M.; May, A.M.; Slebodnick, C.; et al. A New Class of Metal-Cyclam-Based Zirconium Metal–Organic Frameworks for CO2 Adsorption and Chemical Fixation. J. Am. Chem. Soc. 2018, 140, 993–1003. [Google Scholar] [CrossRef]
- Epp, K.; Semrau, A.L.; Cokoja, M.; Fischer, R.A. Dual Site Lewis-Acid Metal-Organic Framework Catalysts for CO2 Fixation: Counteracting Effects of Node Connectivity, Defects and Linker Metalation. ChemCatChem 2018, 10, 3506–3512. [Google Scholar] [CrossRef]
- Lu, B.-B.; Yang, J.; Liu, Y.-Y.; Ma, J.-F. A Polyoxovanadate–Resorcin[4]arene-Based Porous Metal–Organic Framework as an Efficient Multifunctional Catalyst for the Cycloaddition of CO2 with Epoxides and the Selective Oxidation of Sulfides. Inorg. Chem. 2017, 56, 11710–11720. [Google Scholar] [CrossRef]
- Suzuki, M.; Sugai, T. Mechanistic Studies on Nitrosation–Deaminocyclization of Mono-Carbamoylated Vicinal Amino Alcohols and Diols: A New Preparative In Situ Formation of Ethanediazo Hydroxide for the Ethylation of Carboxylates under Mild Conditions. Bull. Chem. Soc. Jpn. 2004, 77, 1217–1227. [Google Scholar] [CrossRef]
- Itaya, T.; Iida, T.; Natsutani, I.; Ohba, M. Reactions of Oxalyl Chloride with 1,2-Cycloalkanediols in the Presence of Triethylamine. Chem. Pharm. Bull. 2002, 50, 83–86. [Google Scholar] [CrossRef]


 | |||
|---|---|---|---|
| Entry | Catalyst | Cocatalyst | Conversion (%) b |
| 1 | ZnI2/tBuN=C(NMe2)2 | none | 26 |
| 2 | ZnI2/tBuN=C(NMe2)2 | ZnCl2 | 81 |
| 3 | tBuN=C(NMe2)2 | ZnCl2 | 1 |
| 4 | none | ZnCl2 | 0 |
 | |||||||
| Combination A: ZnI2/tBuN=C(NMe2)2 + metal chloride | |||||||
| Metal chloride | NbCl5 | ZnCl2 | ZrCl4 | BiCl3 | FeCl3 | MgCl2 | CuCl2 |
| Conversion b (%) | 83 | 81 | 75 | 75 | 73 | 71 | 41 |
| Combination B: ZnI2/base + NbCl5 | |||||||
| Base c | MTBD | DBU | tBuG | nBuG | TBD | PhG | 1,2-dimethylimidazole |
| Conversion b (%) | 86 | 84 | 83 | 83 | 80 | 65 | 22 |
| Combination C: Metal iodide (bromide)/MTBD + NbCl5 | |||||||
| Metal iodide (bromide) | ZnI2 | SnI4 | TiI4 | BiI3 | ZnBr2 | ||
| Conversion b (%) | 86 | 80 | 55 | 50 | 44 | ||
 | |
|---|---|
| Catalyst b | Conversion c (%) |
| ZnI2/MTBD + NbCl5 d | 86 |
| ZnI2-MTBD-NbCl5 e | 86 |
 | ||||
|---|---|---|---|---|
| Entry | R1 | R2 | 2 | Yield (%) b |
| 1 | Ph | H | 2a | 95 |
| 2 | Me | H | 2b | 90 |
| 3 c | CH2OPh | H | 2c | 92 |
| 4 | CH2Cl | H | 2d | 85 |
| 5 c | C8H15 | H | 2e | 89 |
| 6 | CH2OCH2CH=CH2 | H | 2f | 89 |
| 7 d |  | H | 2g | 95 |
| 8 e | (CH2) 4 | 2h | 38 f | |
| 9 e | Ph (trans) | Ph | 2i | 53 g |
 | ||||
| Catalyst A: ZnI2-NbCl5-MTBD b | ||||
| Cycle | 1 | 2 | 3 | |
| Yield (%) | 84 | 67 | 68 | |
| Catalyst B: ZnI2-ZnCl2-DBU c | ||||
| Cycle | 1 | 2 | 3 | 4 |
| Yield (%) | 78 | 81 | 85 | 83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihara, M.; Nakao, S.; Nakai, T.; Mizuno, T. Zinc Iodide-Metal Chloride-Organic Base: An Efficient Catalytic System for Synthesis of Cyclic Carbonates from Carbon Dioxide and Epoxides under Ambient Conditions. Catalysts 2023, 13, 1214. https://doi.org/10.3390/catal13081214
Mihara M, Nakao S, Nakai T, Mizuno T. Zinc Iodide-Metal Chloride-Organic Base: An Efficient Catalytic System for Synthesis of Cyclic Carbonates from Carbon Dioxide and Epoxides under Ambient Conditions. Catalysts. 2023; 13(8):1214. https://doi.org/10.3390/catal13081214
Chicago/Turabian StyleMihara, Masatoshi, Shuichi Nakao, Takeo Nakai, and Takumi Mizuno. 2023. "Zinc Iodide-Metal Chloride-Organic Base: An Efficient Catalytic System for Synthesis of Cyclic Carbonates from Carbon Dioxide and Epoxides under Ambient Conditions" Catalysts 13, no. 8: 1214. https://doi.org/10.3390/catal13081214
APA StyleMihara, M., Nakao, S., Nakai, T., & Mizuno, T. (2023). Zinc Iodide-Metal Chloride-Organic Base: An Efficient Catalytic System for Synthesis of Cyclic Carbonates from Carbon Dioxide and Epoxides under Ambient Conditions. Catalysts, 13(8), 1214. https://doi.org/10.3390/catal13081214