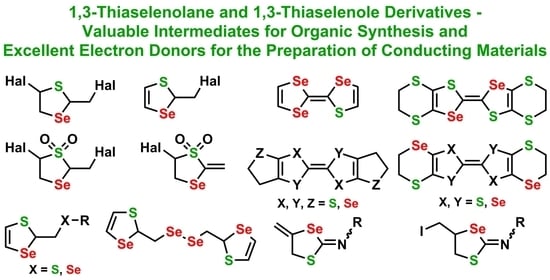
Recent Advances in Design and Synthesis of 1,3-Thiaselenolane and 1,3-Thiaselenole Derivatives
Abstract
:1. Introduction


2. 1,3-Thiaselenolane and 1,3-Thiaselenole Derivatives
2.1. 1,3-Thiaselenolane and 1,3-Thiaselenole Derivatives Based on Selenium Dihalides
2.2. 1,3-Thiaselenolanes Based on Catalytic Reactions
2.3. Oxidized Derivatives of 1,3-Thiaselenolane and 1,3-Thiaselenole
2.4. 1,4,5,8-Diselenadithiafulvalene Derivatives
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santi, C. (Ed.) Organoselenium Chemistry: Between Synthesis and Biochemistry; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014. [Google Scholar] [CrossRef]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2129. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.; Koketsu, M. Recent developments in the synthesis of biologically relevant selenium-containing scaffolds. Coord. Chem. Rev. 2017, 339, 104–127. [Google Scholar] [CrossRef]
- Devillanova, F.A.; du Mont, W.-W. (Eds.) Handbook of Chalcogen Chemistry: New Perspectives in Sulfur, Selenium and Tellurium, 2nd ed.; Royal Society of Chemistry Publishing: Cambridge, UK, 2013. [Google Scholar] [CrossRef]
- Rappoport, Z. (Ed.) PATAI’s Chemistry of Functional Groups: The Chemistry of Organic Selenium and Tellurium Compounds; John Wiley and Sons, Inc.: Chichester, UK, 2013. [Google Scholar] [CrossRef]
- Sarma, B.K.; Mugesh, G. Glutathione peroxidase (GPx)-like antioxidant activity of the organoselenium drug ebselen: Unexpected complications with thiol exchange reactions. J. Am. Chem. Soc. 2005, 127, 11477–11485. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, K.; Shah, P.; Singh, H.B.; Butcher, R.J. Synthesis, structure, and glutathione peroxidase-like activity of amino acid containing ebselen analogues and diaryl diselenides. Chem. Eur. J. 2011, 17, 12741–12755. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A. Click chemistry of selenium dihalides: Novel bicyclic organoselenium compounds based on selenenylation/bis-functionalization reactions and evaluation of glutathione peroxidase-like activity. Int. J. Mol. Sci. 2022, 23, 15629. [Google Scholar] [CrossRef] [PubMed]
- Musalov, M.V.; Kapustina, I.S.; Spiridonova, E.V.; Ozolina, N.V.; Amosova, S.V.; Borodina, T.N.; Potapov, V.A. Transannular Selenocyclofunctionalization of 1,5-cyclooctadiene: The Antioxidant Properties of 9-selenabicyclo [3.3.1]nonane Derivatives and the Discovery of Increasing Both GPx and GR Activities. Inorganics 2023, 11, 304. [Google Scholar] [CrossRef]
- Elsherbini, M.; Hamama, W.S.; Zoorob, H.H. Recent advances in the chemistry of selenium-containing heterocycles: Five-membered ring systems. Coord. Chem. Rev. 2016, 312, 149–177. [Google Scholar] [CrossRef]
- Scheide, M.R.; Schneider, A.R.; Jardim, G.A.M.; Martins, G.M.; Durigon, D.C.; Saba, S.; Rafique, J.; Braga, A.L. Electrochemical synthesis of selenyl-dihydrofurans via anodic selenofunctionalization of allyl-naphthol/phenol derivatives and their anti-Alzheimer activity. Org. Biomol. Chem. 2020, 18, 4916–4921. [Google Scholar] [CrossRef]
- Barbosa, F.A.R.; Canto, R.F.S.; Teixeira, K.F.; de Souza, A.S.; de Oliveira, A.S.; Braga, A.L. Selenium-derivative compounds: A review of new perspectives in the treatment of Alzheimer’s disease. Curr. Med. Chem. 2023, 30, 689–700. [Google Scholar] [CrossRef]
- Kharma, A.; Jacob, C.; Bozzi, Í.A.O.; Jardim, G.A.M.; Braga, A.L.; Salomão, K.; Gatto, C.C.; Silva, M.F.S.; Pessoa, C.; Stangier, M.; et al. Electrochemical selenation/cyclization of quinones: A rapid, green and efficient access to functionalized trypanocidal and antitumor compounds. Eur. J. Org. Chem. 2020, 29, 4474–4486. [Google Scholar] [CrossRef]
- Álvarez-Pérez, M.; Ali, W.; Marć, M.A.; Handzlik, J.; Domínguez-Álvarez, E. Selenides and diselenides: A review of their anticancer and chemopreventive activity. Molecules 2018, 23, 628. [Google Scholar] [CrossRef] [PubMed]
- Al-Balas, Q.A.; Al-Smadi, M.L.; Hassan, M.A.; Al Jabal, G.A.; Almaaytah, A.M.; Alzoubi, K.H. Multi-armed 1,2,3-selenadiazole and 1,2,3-thiadiazole benzene derivatives as novel glyoxalase-I inhibitors. Molecules 2019, 24, 3210. [Google Scholar] [CrossRef] [PubMed]
- Krasowska, D.; Iraci, N.; Santi, C.; Drabowicz, J.; Cieslak, M.; Kaźmierczak-Barańska, J.; Palomba, M.; Królewska-Golińska, K.; Magiera, J.; Sancineto, L. Diselenides and benzisoselenazolones as antiproliferative agents and glutathione-S-transferase inhibitors. Molecules 2019, 24, 2914. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Luo, C.; Zhang, X.; Guo, M.; Sun, M.; Yu, H.; Chen, Q.; Yang, W.; Wang, M.; Zuo, S.; et al. Probing the impact of sulfur/selenium/carbon linkages on prodrug nanoassemblies for cancer therapy. Nat. Commun. 2019, 10, 3211. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lai, H.; Hou, L.; Chen, T. Rational design and action mechanisms of chemically innovative organoselenium in cancer therapy. Chem. Commun. 2020, 56, 179–196. [Google Scholar] [CrossRef]
- Benassi, J.C.; Barbosa, F.A.R.; Candiotto, G.; Grinevicius, V.M.A.S.; Filho, D.W.; Braga, A.L.; Pedrosa, R.C. Docking and molecular dynamics predicted B-DNA and dihydropyrimidinone selenoesters interactions elucidating antiproliferative effects on breast adenocarcinoma cells. J. Biomol. Struct. Dyn. 2022, 40, 8261–8273. [Google Scholar] [CrossRef]
- dos Santos, D.C.; Rafique, J.; Saba, S.; Almeida, G.M.; Siminski, T.; Pádua, C.; Filho, D.W.; Zamoner, A.; Braga, A.L.; Pedrosa, R.C.; et al. Apoptosis oxidative damage-mediated and antiproliferative effect of selenylated imidazo [1,2-a]pyridines on hepatocellular carcinoma HepG2 cells and in vivo. J. Biochem. Mol. Toxicol. 2021, 35, e22663. [Google Scholar] [CrossRef]
- Benassi, J.C.; Barbosa, F.A.R.; Grinevicius, V.M.A.S.; Ourique, F.; Coelho, D.; Felipe, K.B.; Braga, A.L.; Filho, D.W.; Pedrosa, R.C. Novel dihydropyrimidinone-derived selenoesters as potential cytotoxic agents to human hepatocellular carcinoma: Molecular docking and DNA fragmentation. Anti-Cancer Agents Med. Chem. 2021, 21, 703–715. [Google Scholar] [CrossRef]
- Casaril, A.M.; Domingues, M.; Bampi, S.R.; de Andrade Lourenço, D.; Padilha, N.B.; Lenardão, E.J.; Sonego, M.; Seixas, F.K.; Collares, T.; Nogueira, C.W.; et al. The selenium-containing compound 3-((4-chlorophenyl)selanyl)-1-methyl-1H-indole reverses depressive-like behavior induced by acute restraint stress in mice: Modulation of oxido-nitrosative stress and inflammatory pathway. Psychopharmacology 2019, 236, 2867–2880. [Google Scholar] [CrossRef]
- Besckow, E.M.; Nonemacher, N.T.; Garcia, C.S.; da Silva Espindola, C.N.; Balbom, E.B.; Gritzenco, F.; Savegnago, L.; Godoi, B.; Bortolatto, C.F.; Brüning, C.A. Antidepressant-like effect of a selenopropargylic benzamide in mice: Involvement of the serotonergic system. Psychopharmacology 2020, 237, 3149–3159. [Google Scholar] [CrossRef]
- da Silva Teixeira Rech, T.; Alves, A.G.; Strelow, D.N.; Krüger, L.D.; Carraro Júnior, L.R.; dos Santos Neto, J.S.; Braga, A.L.; Brüning, C.A.; Bortolatto, C.F. 2-Phenyl-3-(phenylselanyl)benzofuran elicits acute antidepressant-like action in male Swiss mice mediated by modulation of the dopaminergic system and reveals therapeutic efficacy in both sexes. Psychopharmacology 2021, 238, 3013–3024. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, T.B.; Bilheri, F.N.; Zeni, G.R.; Nogueira, C.W. Dopaminergic system contribution to the antidepressant-like effect of 3-phenyl-4-(phenylseleno) isoquinoline in mice. Behav. Brain Res. 2020, 386, 112602. [Google Scholar] [CrossRef] [PubMed]
- Gall, J.I.; Alves, A.G.; Carraro Júnior, L.R.; da Silva Teixeira Rech, T.; dos Santos Neto, J.S.; Alves, D.; Pereira Soares, M.S.; Spohr, L.; Spanevello, R.M.; Brüning, C.A.; et al. Insights into serotonergic and antioxidant mechanisms involved in antidepressant-like action of 2-phenyl-3-(phenylselanyl)benzofuran in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109956. [Google Scholar] [CrossRef]
- Jain, V.K.; Priyadarsini, K.I. (Eds.) Organoselenium Compounds in Biology and Medicine: Synthesis, Biological and Therapeutic Treatments; Royal Society of Chemistry Publishing: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Sarturi, J.M.; Dornelles, L.; Segatto, N.V.; Collares, T.; Seixas, F.K.; Piccoli, B.C.; da Silva, F.D.; Omage, F.B.; da Rocha, J.B.T.; Balaguez, R.A.; et al. Chalcogenium-AZT derivatives: A plausible strategy to tackle the RT-inhibitors-related oxidative stress while maintaining their anti-HIV properties. Curr. Med. Chem. 2023, 30, 2449–2462. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Sancineto, L.; Mangiavacchi, F.; Dabrowska, A.; Pacuła, A.; Obieziurska-Fabisiak, M.; Scimmi, C.; Lei, Y.; Kong, J.; Zhao, Y.; dos Santos Machado, K.; et al. Organoselenium mild electrophiles in the inhibition of Mpro and SARSCoV-2 replication. ChemRxiv, 2020; preprint. [Google Scholar] [CrossRef]
- Mangiavacchi, F.; Botwina, P.; Menichetti, E.; Bagnoli, L.; Rosati, O.; Marini, F.; Fonseca, S.F.; Abenante, L.; Alves, D.; Dabrowska, A.; et al. Seleno-functionalization of quercetin improves the non-covalent inhibition of Mpro and its antiviral activity in cells against SARS-CoV-2. Int. J. Mol. Sci. 2021, 22, 7048. [Google Scholar] [CrossRef]
- Davidenko, N.A.; Get’manchuk, Y.P.; Itskovskaya, I.N.; Mokrinskaya, E.V.; Pavlov, V.A.; Chuprina, N.G. Holographic recording media based on polymer compositions with Fe2O3, ZnO, and CdS nanoparticles. J. Appl. Spectrosc. 2005, 72, 893–898. [Google Scholar] [CrossRef]
- Get’Manchuk, Y.P.; Davidenko, I.I.; Davidenko, N.A.; Mokrinskaya, E.V.; Mysyk, D.D.; Mysyk, R.D. Sensitization of photoconductivity in polymeric compositions with Fe2O3 and CdS nanoparticles by an organic compound with intramolecular charge transfer. Theor. Exp. Chem. 2004, 40, 7–11. [Google Scholar] [CrossRef]
- Getmanchuk, Y.P.; Davidenko, N.A.; Davidenko, I.I.; Kunitskaya, L.R.; Kozel, G.I.; Pavlov, V.A.; Chuprina, N.G. Effect of oligomer structure on the diffraction efficiency of holographic recording media. Theor. Exp. Chem. 2015, 51, 104–108. [Google Scholar] [CrossRef]
- Davidenko, N.A.; Get’manchuk, Y.P.; Zabolotnyi, M.A.; Zabolotnaya, T.G.; Linets, L.P.; Mokrinskaya, E.V.; Pavlov, V.A.; Studzinskii, S.L.; Chuprina, N.G. Photosensitivity of holographic recording media based on films of polymer compositions with cadmium sulfide nanoparticles. High Energy Chem. 2006, 40, 410–416. [Google Scholar] [CrossRef]
- Fujiwara, E.; Yamamoto, K.; Shimamura, M.; Zhou, B.; Kobayashi, A.; Takahashi, K.; Okano, Y.; Cui, H.; Kobayashi, H. (nBu4N)[Ni(dmstfdt)2]: A planar nickel coordination complex with an extended-TTF ligand exhibiting metallic conduction, metal-insulator transition, and weak ferromagnetism. Chem. Mater. 2007, 19, 553–558. [Google Scholar] [CrossRef]
- Alberola, A.; Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J. A molecular metal ferromagnet from the organic donor bis(ethylenedithio)tetraselenafulvalene and bimetallic oxalate complexes. J. Am. Chem. Soc. 2003, 125, 10774–10775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Wang, Z.; Gao, S.; Guo, Y.; Liue, F.; Zhua, D. BETS3[Cu2(C2O4)3](CH3OH)2: An organic–inorganic hybrid antiferromagnetic metal (BETS = bisethylene(tetraselenfulvalene)). Cryst. Eng. Comm. 2013, 15, 3529–3535. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kamada, Y.; Sugimoto, T.; Tada, T.; Noguchi, S.; Nakazumi, H.; Shiro, M.; Yoshino, H.; Murata, K. Electrical conducting and magnetic properties of (ethylenedithiotetrathiafulvalenothioquinone-1,3-diselenolemethide)2∙FeBr4(GaBr4) crystals with two different interlayer arrangements of donor molecules. Inorg. Chem. 2003, 42, 5192–5201. [Google Scholar] [CrossRef]
- Wang, M.; Yu, T.; Tan, L.-S.; Urbas, A.M.; Chiang, L.Y. Enhancement of photoswitchable dielectric property by conducting electron donors on plasmonic core-shell gold-fluorenyl C60 nanoparticles. J. Phys. Chem. C 2018, 122, 12512–12523. [Google Scholar] [CrossRef]
- Akutsu, H.; Koyama, Y.; Turner, S.S.; Furuta, K.; Nakazawa, Y. Structures and properties of new organic conductors: BEDT-TTF, BEST and BETS salts of the HOC2H4SO3– anion. Crystals 2020, 10, 775. [Google Scholar] [CrossRef]
- Kobayashi, T.; Taniguchi, H.; Ohnuma, A.; Kawamoto, A. Unconventional superconductivity in λ-(BETS)2GaCl4 probed by 13C NMR. Phys. Rev. B 2020, 102, 121106. [Google Scholar] [CrossRef]
- Naka, K.; Uemura, T.; Chujo, Y. π-Conjugated poly(dithiafulvene)s and poly(diselenafulvene)s: Effects of side alkyl chains on optical, electrochemical, and conducting properties. Macromolecules 2002, 35, 3539–3543. [Google Scholar] [CrossRef]
- Vlasova, R.M.; Drichko, N.V.; Petrov, B.V.; Semkin, V.N.; Zhilyaeva, E.I.; Lyubovskaya, R.N.; Olejniczak, I.; Kobayashi, A.; Kobayashi, H. Optical properties of new organic conductors based on the BEDT-TSeF molecule (the κ-(BETS)4Hg2.84Br8 super-conductor and κ-(BETS)4Hg3Cl8 metal) in the range 300–15 K. Phys. Solid State 2004, 46, 1985–1993. [Google Scholar] [CrossRef]
- Anyfantis, G.C.; Papavassiliou, G.C.; Aloukos, P.; Couris, S.; Weng, Y.F.; Yoshino, H.; Murata, K. Unsymmetrical single-component nickel 1,2-dithiolene complexes with extended tetrachalcogenafulvalenedithiolato ligands. Z. Naturforsch. 2007, 62, 200–204. [Google Scholar] [CrossRef]
- Ahluwalia, G.K. (Ed.) Applications of Chalcogenides: S, Se, and Te; Springer International Publishing AG: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Makhaeva, N.A.; Amosova, S.V.; Potapov, V.A. Recent advances in design and synthesis of diselenafulvenes, tetraselenafulvalenes, and their tellurium analogs and application for materials sciences. Molecules 2022, 27, 5613. [Google Scholar] [CrossRef]
- Nagy-Felsobuki, E.; Peel, J.B. Photoelectron spectra of sulfur dibromide and selenium dibromide. Chem. Phys. 1980, 45, 189–194. [Google Scholar] [CrossRef]
- Milne, J. Selenium dibromide and dichloride in acetonitrile. Polyhedron 1985, 4, 65–68. [Google Scholar] [CrossRef]
- Lamoureux, M.; Milne, J. Selenium chloride and bromide equilibria in aprotic solvents; a 77Se NMR study. Polyhedron 1990, 9, 589–595. [Google Scholar] [CrossRef]
- Steudel, R.; Jensen, D.; Baumgart, F. 77Se-NMR and Raman spectroscopic characterization of selenium dibromide (SeBr2) and its reaction with titanocene pentasulphide to give cyclic selenium sulphides of ring size 6, 7, 8, and 12. Polyhedron 1990, 9, 1199–1208. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V. New methods for preparation of organoselenium and organotellurium compounds from elemental chalcogens. Russ. J. Org. Chem. 2003, 39, 1373–1380. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Musalova, M.V.; Amosova, S.V. Recent advances in organochalcogen synthesis based on reactions of chalcogen halides with alkynes and alkenes. Curr. Org. Chem. 2016, 20, 136–145. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A. Selenium dihalides: New possibilities for the synthesis of selenium-containing heterocycles. Chem. Heterocycl. Comp. 2017, 53, 150–152. [Google Scholar] [CrossRef]
- Abakumov, G.A.; Piskunov, A.V.; Cherkasov, V.K.; Fedushkin, I.L.; Ananikov, V.P.; Eremin, D.B.; Gordeev, E.G.; Beletskaya, I.P.; Averin, A.D.; Bochkarev, M.N.; et al. Organoelement chemistry: Promising growth areas and challenges. Russ. Chem. Rev. 2018, 87, 393–507. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Maylyan, A.A.; Khabibulina, A.G.; Zinchenko, S.V.; Amosova, S.V. Selenium dihalides click chemistry: Highly efficient stereoselective addition to alkynes and evaluation of glutathione peroxidase-like activity of bis(E-2-halovinyl) selenides. Molecules 2022, 27, 1050. [Google Scholar] [CrossRef] [PubMed]
- Musalov, M.V.; Yakimov, V.A.; Potapov, V.A.; Amosova, S.V.; Borodina, T.N.; Zinchenko, S.V. A novel methodology for the synthesis of condensed selenium heterocycles based on the annulation and annulation–methoxylation reactions of selenium dihalides. New J. Chem. 2019, 43, 18476–18483. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Amosova, S.V. Reactions of selenium dichloride and dibromide with unsaturated ethers. Annulation of 2,3-dihydro-1,4-oxaselenine to the benzene ring. Tetrahedron Lett. 2011, 52, 4606–4610. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Khabibulina, A.G.; Maylyan, A.A.; Borodina, T.N.; Zinchenko, S.V.; Amosova, S.V. Regioselective one-pot synthesis of novel functionalized organoselenium compound by bis-alkoxyselenenylation of alkenes with selenium dibromide and alcohols. Inorganics 2022, 10, 239. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V. Triple-click chemistry of selenium dihalides: Catalytic regioselective and highly efficient synthesis of bis-1,2,3-triazole derivatives of 9-selenabicyclo [3.3.1]nonane. Catalysts 2022, 12, 1032. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Yakimov, V.A.; Musalova, M.V.; Maylyan, A.A.; Zinchenko, S.V.; Amosova, S.V. A Regioselective synthesis of novel functionalized organochalcogen compounds by chalcogenocyclofunctionalization reactions based on chalcogen halides and natural products. Molecules 2021, 26, 3729. [Google Scholar] [CrossRef]
- Amosova, S.V.; Filippov, A.S.; Makhaeva, N.A.; Albanov, A.I.; Potapov, V.A. Green organoselenium chemistry: Selective syntheses of new 1,4-thiaselenine derivatives based on reactions of thiaselenole reagent with alcohols and water. Inorganics 2023, 11, 281. [Google Scholar] [CrossRef]
- Amosova, S.V.; Penzik, M.V.; Albanov, A.I.; Potapov, V.A. The reaction of selenium dichloride with divinyl sulfide. J. Organomet. Chem. 2009, 694, 3369–3372. [Google Scholar] [CrossRef]
- Amosova, S.V.; Penzik, M.V.; Potapov, V.A.; Albanov, A.I. A reaction of selenium dichloride with divinyl sulfide. Russ. Chem. Bull. 2008, 57, 1323. [Google Scholar] [CrossRef]
- Potapov, V.A.; Shagun, V.A.; Penzik, M.V.; Amosova, S.V. Quantum chemical studies of the reaction of selenium dichloride with divinyl sulfide and comparison with experimental results. J. Organomet. Chem. 2010, 695, 1603–1608. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Krivdin, L.B.; Potapov, V.A.; Penzik, M.V.; Amosova, S.V. Conformational analysis and diastereotopic assignments in the series of selenium-containing heterocycles by means of 77Se- 1H spin-spin coupling constants: A combined theoretical and experimental study. Magn. Reson. Chem. 2011, 49, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Trofimov, B.A.; Amosova, S.V. Divinyl sulfide: Synthesis, properties, and applications. Sulfur Rep. 1984, 3, 323–393. [Google Scholar] [CrossRef]
- Amosova, S.V.; Gavrilova, G.M. Divinyl sulfide and its selenium and tellurium analogs as starting materials for the preparation of polyfunctional alkyl, aromatic, and heteroaromatic vinyl chalcogenides. Heteroatom Chem. 2006, 17, 491–498. [Google Scholar] [CrossRef]
- Amosova, S.V.; Penzik, M.V.; Albanov, A.I.; Potapov, V.A. Reaction of selenium dibromide with divinyl sulfide. Russ. J. Gen. Chem. 2009, 79, 161. [Google Scholar] [CrossRef]
- Amosova, S.V.; Penzik, M.V.; Albanov, A.I.; Potapov, V.A. Addition of selenium dibromide to divinyl sulfide: Spontaneous rearrangement of 2,6-dibromo-1,4-thiaselenane to 5-bromo-2-bromomethyl-1,3-thiaselenolane. Tetrahedron Lett. 2009, 50, 306–308. [Google Scholar] [CrossRef]
- Amosova, S.V.; Penzik, M.V.; Potapov, V.A.; Filippov, A.S.; Shagun, V.A.; Albanov, A.I.; Borodina, T.N.; Smirnov, V.I. Unexpected regioselective reactions of 2-bromomethyl-1,3-thiaselenole with dithiocarbamates: The first example of nucleophilic attack at selenium atom of seleniranium intermediate. Synlett 2016, 27, 1653–1658. [Google Scholar] [CrossRef]
- Amosova, S.V.; Novokshonova, I.A.; Penzik, M.V.; Filippov, A.S.; Albanov, A.I.; Potapov, V.A. Reaction of 2-bromomethyl-1,3-thiaselenole with thiourea: En route to the first representatives of 2-(organylsulfanyl)-2,3-dihydro-1,4-thiaselenines. Tetrahedron Lett. 2017, 58, 4381–4383. [Google Scholar] [CrossRef]
- Tachibana, Y. (Hydroxyalkyl)pyridines as bifunctional catalysts. Dehydrobromination of 7-bromocholesterol. Bull. Chem. Soc. Jpn. 1978, 51, 3085–3086. [Google Scholar] [CrossRef]
- Accurso, A.A.; Cho, S.-H.; Amin, A.; Potapov, V.A.; Amosova, S.V.; Finn, M.G. Thia-, aza-, and selena [3.3.1]bicyclononane dichlorides: Rates vs internal nucleophile in anchimeric assistance. J. Org. Chem. 2011, 76, 4392–4395. [Google Scholar] [CrossRef]
- Amosova, S.V.; Penzik, M.V.; Potapov, V.A.; Albanov, A.I. Unexpected reaction of 2-(bromomethyl)-1,3-thiaselenole with ammonium thiocyanate. Russ. J. Org. Chem. 2015, 51, 287–289. [Google Scholar] [CrossRef]
- Potapov, V.A.; Filippov, A.S.; Amosova, S.V. Selective synthesis of 1,3-thiaselenol-2-ylmethyl selenocyanate. Russ. J. Org. Chem. 2018, 54, 957–958. [Google Scholar] [CrossRef]
- Amosova, S.V.; Filippov, A.A.; Makhaeva, N.A.; Albanov, A.I.; Potapov, V.A. Regio- and stereoselective synthesis of new ensembles of diversely functionalized 1,3-thiaselenol-2-ylmethyl selenides by a double rearrangement reaction. Beilstein J. Org. Chem. 2020, 16, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Amosova, S.V.; Filippov, A.S.; Makhaeva, N.A.; Albanov, A.I.; Potapov, V.A. New methodology of nucleophilic substitution at three different centers of a seleniranium intermediate in reactions of 2-bromomethyl-1,3-thiaselenole with mercapto benzazoles. New J. Chem. 2019, 43, 11189–11199. [Google Scholar] [CrossRef]
- Amosova, S.V.; Shagun, V.A.; Makhaeva, N.A.; Novokshonova, I.A.; Potapov, V.A. Quantum chemical and experimental studies of an unprecedented reaction pathway of nucleophilic substitution of 2-bromomethyl-1,3-thiaselenole with 1,3-benzothiazole-2-thiol proceeding stepwise at three different centers of seleniranium intermediates. Molecules 2021, 26, 6685. [Google Scholar] [CrossRef]
- Wang, Z.; Yamazaki, S.; Mikata, Y.; Oba, M.; Takashima, H.; Morimoto, T.; Ogawa, A. Intramolecular Diels–Alder reactions of α-bromostyrene functionalized unsaturated carboxamides. J. Org. Chem. 2022, 87, 11148–11164. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Lai, Y.; Li, H.; Ding, J.; Yao, H.; Su, Q.; Huang, B.; Ouyang, M.-A.; Tong, R. Environmentally benign and user-friendly in situ generation of nitrile imines from hydrazones for 1,3-dipolar cycloaddition. J. Org. Chem. 2022, 87, 10550–10554. [Google Scholar] [CrossRef]
- Ganin, A.S.; Moskalik, M.Y.; Garagan, I.A.; Astakhova, V.V.; Shainyan, B.A. Triflamidation of allyl-containing substances: Unusual dehydrobromination vs. intramolecular heterocyclization. Molecules 2022, 27, 6910. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, Z. An efficient synthesis of 5α-androst-1-ene-3,17-dione. Steroids 2006, 71, 1088–1090. [Google Scholar] [CrossRef]
- Forti, L.; Ghelfi, F.; Pagnoni, U.M. Stereoselective dehydrobromination of alkyl α-Br-α-Cl-carboxylates. Tetrahedron Lett. 1995, 36, 3023–3026. [Google Scholar] [CrossRef]
- Abrigach, F.; Rokni, Y.; Takfaoui, A.; Khoutoul, M.; Doucet, H.; Asehraou, A.; Touzani, R. In vitro screening, homology modeling and molecular docking studies of some pyrazole and imidazole derivatives. Biomed. Pharmacother. 2018, 103, 653–661. [Google Scholar] [CrossRef]
- Abbady, M.A.; Kandeel, M.M.; Abdel-Hafez, S.H.; Abou-Omar, M.-A.M. Organic Selenium Compounds, Part IV: Synthesis and applications of some new diaryl selenides containing azomethine and oxazole moieties. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 1708–1725. [Google Scholar] [CrossRef]
- Amosova, S.V.; Filippov, A.S.; Potapov, V.A.; Makhaeva, N.A.; Albanov, A.I. Unexpected regioselective reactions of 2-(bromomethyl)-1,3-thiaselenole with 1-methyl-1h-imidazol-2-thiol, accompanied by rearrangements. Russ. J. Org. Chem. 2018, 54, 1697–1701. [Google Scholar] [CrossRef]
- Amosova, S.V.; Martynov, A.V.; Albanov, A.I.; Potapov, V.A. Synthesis of novel (2,3-dihydro-1,4-thiaselenin-2-yl)sulfanyl-substituted pharmacophoric nitrogen heterocycles based on 2-(bromomethyl)-1,3-thiaselenole. Chem. Heterocycl. Compd. 2020, 56, 226–232. [Google Scholar] [CrossRef]
- Brittain, J.; Gareau, Y. Triphenylsilanethiol: A solid H2S equivalent in the ring opening of epoxides. Tetrahedron. Lett. 1993, 34, 3363–3366. [Google Scholar] [CrossRef]
- Tanini, D.; Tiberi, C.; Gellini, C.; Salvi, P.R.; Capperucci, A. A straightforward access to stable β-functionalized alkyl selenols. Adv. Synth. Catal. 2018, 360, 3367–3375. [Google Scholar] [CrossRef]
- Degl’Innocenti, A.; Capperucci, A.; Cerreti, A.; Pollicino, S.; Scapecchi, S.; Malesci, I.; Castagnoli, G. Regio- and enantioselective ring-opening of epoxides with HMDST: A straightforward access to 1,2-mercaptoalcohols. Synlett 2005, 20, 3063–3066. [Google Scholar] [CrossRef]
- Degl’Innocenti, A.; Capperucci, A.; Castagnoli, G.; Malesci, I.; Tiberi, C.; Innocenti, B. Selenosilanes mediated stereoselective synthesis of polyfunctionalized organic molecules. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 966–969. [Google Scholar] [CrossRef]
- Xia, S.; Wang, X.; Ge, Z.-M.; Cheng, T.-M.; Li, R.-T. An efficient synthesis of aryldithiocarbamic acid esters from Michael addition of electron-deficient alkenes with arylamines and CS2 in solid media alkaline Al2O3. Tetrahedron 2009, 65, 1005–1009. [Google Scholar] [CrossRef]
- Bartoli, G.; Bartolacci, M.; Giuliani, A.; Marcantoni, E.; Massaccesi, M.; Torregiani, E. Improved heteroatom nucleophilic addition to electron-poor alkenes promoted by CeCl3∙7H2O/NaI system supported on alumina in solvent-free conditions. J. Org. Chem. 2005, 70, 169–174. [Google Scholar] [CrossRef]
- Lenardao, E.J.; Trecha, D.O.; Ferreira, P.C.; Jacob, R.G.; Perin, G. Green Michael addition of thiols to electron deficient alkenes using KF/alumina and recyclable solvent or solvent-free conditions. J. Braz. Chem. Soc. 2009, 20, 93–99. [Google Scholar] [CrossRef]
- Yang, L.; Xu, L.-W.; Xia, C.-G. Highly efficient KF/Al2O3-catalyzed versatile hetero-Michael addition of nitrogen, oxygen, and sulfur nucleophiles to α,β-ethylenic compounds. Tetrahedron Lett. 2005, 46, 3279–3282. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Bardajee, G.R.; Chadorneshine Veranlou, R.O. KF/Al2O3-mediated Michael addition of thiols to electron-deficient olefins. Synth. Commun. 2005, 35, 2427–2433. [Google Scholar] [CrossRef]
- Cheng, S.; Comer, D.D. An alumina-catalyzed Michael addition of mercaptans to N-anilinomaleimides and its application to the solution-phase parallel synthesis of libraries. Tetrahedron Lett. 2002, 43, 1179–1181. [Google Scholar] [CrossRef]
- Tanini, D.; Scarpelli, S.; Ermini, E.; Capperucci, A. Seleno-Michael reaction of stable functionalised alkyl selenols: A versatile tool for the synthesis of acyclic and cyclic unsymmetrical alkyl and vinyl selenides. Adv. Synth. Catal. 2019, 361, 2337–2346. [Google Scholar] [CrossRef]
- Perin, G.; Lenardão, E.J.; Jacob, R.G.; Panatieri, R.B. Synthesis of vinyl selenides. Chem. Rev. 2009, 109, 1277–1301. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Shi, Z.; Liu, Z.; Luan, B.; Zhu, J.; Xue, Y. Synthesis of (E)-β-selenovinyl sulfones through a multicomponent regio- and stereospecific selenosulfonation of alkynes with insertion of sulfur dioxide. Org. Lett. 2018, 20, 6687–6690. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.L.; Alves, E.F.; Silveira, C.C.; de Andrade, L.H. Stereoselective addition of sodium organyl chalcogenolates to alkynylphosphonates: Synthesis of diethyl 2-(organyl)-2-(organochalcogenyl)vinylphosphonates. Tetrahedron Lett. 2000, 41, 161–163. [Google Scholar] [CrossRef]
- Frederickson, M.; Grigg, R. Electrophile mediated heteroatom cyclizations onto C-C π-bonds. Part 1: Halogen and chalcogen mediated cyclization. Org. Prep. Proced. Int. 1997, 29, 33–62. [Google Scholar] [CrossRef]
- da Silva, F.M.; Jones, J., Jr.; de Mattos, M.C.S. A centennial methodology for the preparation of heterocyclic compounds: The intramolecular coiodination of alkenes with internal oxygenated nucleophiles. Curr. Org. Synth. 2005, 2, 393–414. [Google Scholar] [CrossRef]
- Mphahlele, M.J. Molecular iodine-mediated cyclization of tethered heteroatom-containing alkenyl or alkynyl systems. Molecules 2009, 14, 4814–4837. [Google Scholar] [CrossRef] [PubMed]
- Parvatkar, P.T.; Parameswaran, P.S.; Tilve, S.G. Recent developments in the synthesis of five- and six-membered heterocycles using molecular iodine. Chem. Eur. J. 2012, 18, 5460–5489. [Google Scholar] [CrossRef] [PubMed]
- Halimehjani, A.Z.; Maleki, H.; Saidi, M.R. Regiospecific iodocyclization of S-allyl dithiocarbamates: Synthesis of 2-imino-1,3-dithiolane and 2-iminium-1,3-dithiolane derivatives. Tetrahedron Lett. 2009, 50, 2747–2749. [Google Scholar] [CrossRef]
- Koketsu, M.; Kiyokuni, T.; Sakai, T.; Ando, H.; Ishihara, H. Synthesis of 1,3-selenazines and 1,3-selenazolidines via intramolecular addition of N-allylselenoureas. Chem. Lett. 2006, 35, 626–627. [Google Scholar] [CrossRef]
- Garud, D.R.; Koketsu, M. Synthesis of 3-selena-1-dethiacephems and selenazepines via iodocyclization. Org. Lett. 2008, 10, 3319–3322. [Google Scholar] [CrossRef] [PubMed]
- Garud, D.R.; Makimura, M.; Ando, H.; Ishihara, H.; Koketsu, M. First regioselective iodocyclization of O-allylselenocarbamates. Tetrahedron Lett. 2007, 48, 7764–7768. [Google Scholar] [CrossRef]
- Fernandez-Bolanos, J.G.; Lopez, O.; Ulgar, V.; Maya, I.; Fuentes, J. Synthesis of O-unprotected glycosyl selenoureas. A new access to bicyclic sugar isoureas. Tetrahedron Lett. 2004, 45, 4081–4084. [Google Scholar] [CrossRef]
- Toyoda, Y.; Koketsu, M. Synthesis and Z/E isomerization of 2-imino-1,3-thiaselenolanes via iodocyclization. Tetrahedron 2012, 68, 10496–10501. [Google Scholar] [CrossRef]
- Potapov, V.A.; Kurkutov, E.O.; Amosova, S.V. Stereoselective synthesis of 5-bromo-2-bromomethyl-1,3-thiaselenolane 1,1-dioxide by addition of selenium dibromide to divinyl sulfone. Russ. J. Gen. Chem. 2010, 80, 1220–1221. [Google Scholar] [CrossRef]
- Potapov, V.A.; Kurkutov, E.O.; Musalov, M.V.; Amosova, S.V. Reactions of selenium dichloride and dibromide with divinyl sulfone: Synthesis of novel four- and five-membered selenium heterocycles. Tetrahedron Lett. 2010, 51, 5258–5261. [Google Scholar] [CrossRef]
- Potapov, V.A.; Kurkutov, E.O.; Amosova, S.V.; Rusakov, Y.Y. Regioselective dehydrobromination reaction of 5-bromo-2-bromomethyl-1,3-thiaselenolane 1,1-dioxide. Russ. Chem. Bull. 2011, 60, 196–197. [Google Scholar] [CrossRef]
- Starosotnikov, A.M.; Ilkov, K.V.; Bastrakov, M.A.; Fedyanin, I.V.; Kokorekin, V.A. Mild and efficient addition of carbon nucleophiles to condensed pyridines: Influence of structure and limits of applicability. Chem. Heterocycl. Compd. 2020, 56, 92–100. [Google Scholar] [CrossRef]
- Potaczek, P.; Młochowsky, J. From o-substituted bromoselenobenzenes to selenaheterocycles. Pol. J. Chem. 2006, 80, 913–920. [Google Scholar] [CrossRef]
- Iyoda, M.; Watanabe, R.; Miyake, Y. Anomalous ring cleavage of 1,3-dithiole- and 1,3-diselenole-2-thiones under the cross-coupling conditions using triethyl phosphite. Chem. Lett. 2004, 33, 570–571. [Google Scholar] [CrossRef]
- Takimiya, K.; Kataoka, Y.; Nakamura, Y.; Aso, Y.; Otsubo, T. Molecular modifications of methylenedithio-tetraselenafulvalene (MDT-TSF) and methylenedithio-diselenadithiafulvalene (MDT-ST) for superior electron donors. Synthesis 2004, 2004, 1315–1320. [Google Scholar] [CrossRef]
- Nakajima, H.; Katsuhara, M.; Ashizawa, M.; Kawamoto, T.; Mori, T. Ferromagnetic anomaly associated with the antiferromagnetic transitions in (donor)[Ni(mnt)2]-type charge-transfer salts. Inorg. Chem. 2004, 43, 6075–6082. [Google Scholar] [CrossRef]
- Kimura, T. Preparation and electrochemical property of octaethylphthalocyanine fused with four diselenadithiafulvalene units. Heterocycles 2014, 88, 207–212. [Google Scholar] [CrossRef]
- Takimiya, K.; Kodani, M.; Murakami, S.; Otsubo, T.; Aso, Y. Synthesis and properties of ethylenethiotetraselenafulvalene (ET-TSF) and its conductive radical cation salts. Heterocycles 2006, 67, 655–663. [Google Scholar] [CrossRef]
- Kodani, M.; Murakami, S.; Jigami, T.; Takimiya, K.; Aso, Y.; Otsubo, T. Bis(ethylenethio)tetraselenafulvalene and related hybrid diselenadithiafulvalenes as novel electron donors forming highly conductive complexes with 7,7,8,8-tetracyanoquinodimethane. Heterocycles 2001, 54, 225–235. [Google Scholar] [CrossRef]
- Takimiya, K.; Jigami, T.; Kawashima, M.; Kodani, M.; Aso, Y.; Otsubo, T. Synthetic procedure for various selenium-containing electron donors of the bis(ethylenedithio)tetrathiafulvalene (BEDT-TTF) type. J. Org. Chem. 2002, 67, 4218–4227. [Google Scholar] [CrossRef]
- Mori, H.; Suzuki, H.; Okano, T.; Moriyama, H.; Nishio, Y.; Kajita, K.; Kodani, M.; Takimiya, K.; Otsubo, T. Positional order and disorder of symmetric and unsymmetric BEDT-STF salts. J. Solid State Chem. 2002, 168, 626–631. [Google Scholar] [CrossRef]
- Walwyn, R.J.; Chan, B.; Usov, P.M.; Solomon, M.B.; Duyker, S.G.; Koo, J.Y.; Kawano, M.; Turner, P.; Kepert, C.J.; D’Alessandro, D.M. Spectroscopic, electronic and computational properties of a mixed tetrachalcogenafulvalene and its charge transfer complex. J. Mater. Chem. C 2018, 6, 1092–1104. [Google Scholar] [CrossRef]
- Hayashi, T.; Xiao, X.; Yamaji, Y.; Fujiwara, H.; Sugimoto, T.; Nakazumi, H. Novel sulfur–selenium exchange in ethylenedioxy- and ethylenedithiodithiadiselenafulvalenedithiolates. Chem. Lett. 2008, 37, 428–429. [Google Scholar] [CrossRef]
- Hiraoka, T.; Fujiwara, H.; Sugimoto, T.; Li, L.; Weng, Y.; Yokogawa, K.; Murata, K. Magnetic ion salts using selenium analogues of a new donor molecule, benzotetrathiafulvalenothioquinone-1,3-dithiolemethide. J. Low Temp. Phys. 2006, 142, 433–436. [Google Scholar] [CrossRef]





















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amosova, S.V.; Makhaeva, N.A. Recent Advances in Design and Synthesis of 1,3-Thiaselenolane and 1,3-Thiaselenole Derivatives. Catalysts 2023, 13, 1221. https://doi.org/10.3390/catal13081221
Amosova SV, Makhaeva NA. Recent Advances in Design and Synthesis of 1,3-Thiaselenolane and 1,3-Thiaselenole Derivatives. Catalysts. 2023; 13(8):1221. https://doi.org/10.3390/catal13081221
Chicago/Turabian StyleAmosova, Svetlana V., and Nataliya A. Makhaeva. 2023. "Recent Advances in Design and Synthesis of 1,3-Thiaselenolane and 1,3-Thiaselenole Derivatives" Catalysts 13, no. 8: 1221. https://doi.org/10.3390/catal13081221
APA StyleAmosova, S. V., & Makhaeva, N. A. (2023). Recent Advances in Design and Synthesis of 1,3-Thiaselenolane and 1,3-Thiaselenole Derivatives. Catalysts, 13(8), 1221. https://doi.org/10.3390/catal13081221