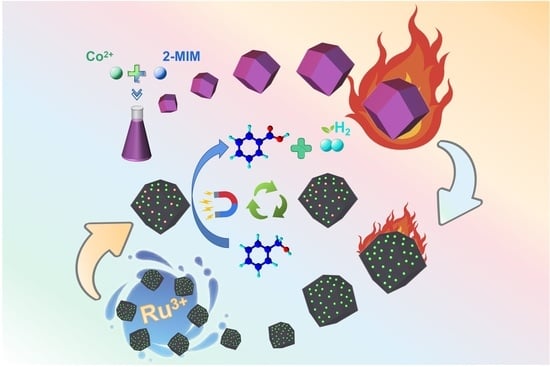
Zeolitic Imidazolium Frameworks-Derived Ru-Based Composite Materials Enable the Catalytic Dehydrogenation of Alcohols to Carboxylic Acids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Rux@Co-NC
2.2. The Catalytic Activity and Recyclability of Rux@Co-NC
2.3. Stability of Ru0.05@Co-NC
3. Experimental Section
3.1. Materials Preparation
3.1.1. Preparation of ZIF-67
3.1.2. Preparation of Co-NC, Co-NC-HCl, and Co-NC-HNO3
3.1.3. Synthesis of Ru@Co-NC, Ru@Co-NC-HCl and Ru@Co-NC-HNO3
3.2. Catalyst Characterization
3.3. Catalytic Reactions
3.4. Catalyst Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvajal-Arroyo, J.M.; Andersen, S.J.; Ganigué, R.; Rozendal, R.A.; Angenent, L.T.; Rabaey, K. Production and extraction of medium chain carboxylic acids at a semi-pilot scale. Chem. Eng. J. 2021, 416, 127886. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, L. alpha-Amino acid N-carboxyanhydride (NCA)-derived synthetic polypeptides for nucleic acids delivery. Adv. Drug Deliv. Rev. 2021, 171, 139–163. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, Y. Directing carboxylic acid dehydrogenation. Science 2021, 374, 1199. [Google Scholar] [CrossRef] [PubMed]
- Tohma, H.; Kita, Y. Hypervalent iodine reagents for the oxidation of alcohols and their application to complex molecule synthesis. Adv. Synth. Catal. 2004, 346, 111–124. [Google Scholar] [CrossRef]
- Patel, S.; Mishra, B.K. Chromium(VI) oxidants having quaternary ammonium ions: Studies on synthetic applications and oxidation kinetics. Tetrahedron 2007, 63, 4367–4406. [Google Scholar] [CrossRef]
- Liu, K.-J.; Jiang, S.; Lu, L.-H.; Tang, L.-L.; Tang, S.-S.; Tang, H.-S.; Tang, Z.; He, W.-M.; Xu, X. Bis(methoxypropyl) ether-promoted oxidation of aromatic alcohols into aromatic carboxylic acids and aromatic ketones with O2 under metal- and base-free conditions. Green Chem. 2018, 20, 3038–3043. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, X.; Li, Y. A new approach for the aerobic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid without using transition metal catalysts. J. Energy Chem. 2018, 27, 243–249. [Google Scholar] [CrossRef]
- Tiburcio, E.; Greco, R.; Mon, M.; Ballesteros-Soberanas, J.; Ferrando-Soria, J.; Lopez-Haro, M.; Carlos Hernandez-Garrido, J.; Oliver-Meseguer, J.; Marini, C.; Boronat, M.; et al. Soluble/MOF-Supported Palladium Single Atoms Catalyze the Ligand-, Additive-, and Solvent-Free Aerobic Oxidation of Benzyl Alcohols to Benzoic Acids. J. Am. Chem. Soc. 2021, 143, 2581–2592. [Google Scholar] [CrossRef]
- Zweifel, T.; Naubron, J.-V.; Gruetzmacher, H. Catalyzed Dehydrogenative Coupling of Primary Alcohols with Water, Methanol, or Amines. Angew. Chem. Int. Ed. 2009, 48, 559–563. [Google Scholar] [CrossRef]
- Trincado, M.; Gruetzmacher, H.; Vizza, F.; Bianchini, C. Domino Rhodium/Palladium-Catalyzed Dehydrogenation Reactions of Alcohols to Acids by Hydrogen Transfer to Inactivated Alkenes. Chem.—Eur. J. 2010, 16, 2751–2757. [Google Scholar] [CrossRef]
- Gianetti, T.L.; Annen, S.P.; Santiso-Quinones, G.; Reiher, M.; Driess, M.; Gruetzmacher, H. Nitrous Oxide as a Hydrogen Acceptor for the Dehydrogenative Coupling of Alcohols. Angew. Chem. Int. Ed. 2016, 55, 1854–1858. [Google Scholar] [CrossRef] [PubMed]
- Balaraman, E.; Khaskin, E.; Leitus, G.; Milstein, D. Catalytic transformation of alcohols to carboxylic acid salts and H2 using water as the oxygen atom source. Nat. Chem. 2013, 5, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Sawama, Y.; Morita, K.; Asai, S.; Kozawa, M.; Tadokoro, S.; Nakajima, J.; Monguchi, Y.; Sajiki, H. Palladium on Carbon-Catalyzed Aqueous Transformation of Primary Alcohols to Carboxylic Acids Based on Dehydrogenation under Mildly Reduced Pressure. Adv. Synth. Catal. 2015, 357, 1205–1210. [Google Scholar] [CrossRef]
- Ghalehshahi, H.G.; Madsen, R. Silver-Catalyzed Dehydrogenative Synthesis of Carboxylic Acids from Primary Alcohols. Chem.—Eur. J. 2017, 23, 11920–11926. [Google Scholar] [CrossRef]
- Monda, F.; Madsen, R. Zinc Oxide-Catalyzed Dehydrogenation of Primary Alcohols into Carboxylic Acids. Chem.—Eur. J. 2018, 24, 17832–17837. [Google Scholar] [CrossRef]
- Yazdani, E.; Heydari, A. Acceptorless dehydrogenative oxidation of primary alcohols to carboxylic acids and reduction of nitroarenes via hydrogen borrowing catalyzed by a novel nanomagnetic silver catalyst. J. Organomet. Chem. 2020, 924, 121453. [Google Scholar] [CrossRef]
- Li, B.; Fang, J.; Xu, D.; Zhao, H.; Zhu, H.; Zhang, F.; Dong, Z. Atomically Dispersed Co Clusters Anchored on N-doped Carbon Nanotubes for Efficient Dehydrogenation of Alcohols and Subsequent Conversion to Carboxylic Acids. ChemSusChem 2021, 14, 4536–4545. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.-Q.; Gong, Y.-Y.; Wang, J.-C.; Yuan, Y.; Cheng, H.; Sang, W.; Chaemchuen, S.; Verpoort, F. Cobalt embedded in nitrogen-doped porous carbon as a robust heterogeneous catalyst for the atom-economic alcohol dehydrogenation to carboxylic acids. Carbon 2021, 174, 284–294. [Google Scholar] [CrossRef]
- Mittal, R.; Awasthi, S.K. Bimetallic Oxide Catalyst for the Dehydrogenative Oxidation Reaction of Alcohols: Practical Application in the Synthesis of Value-Added Chemicals. ACS Sustain. Chem. Eng. 2022, 10, 1702–1713. [Google Scholar] [CrossRef]
- Shit, S.C.; Koley, P.; Joseph, B.; Marini, C.; Nakka, L.; Tardio, J.; Mondal, J. Porous Organic Polymer-Driven Evolution of High-Performance Cobalt Phosphide Hybrid Nanosheets as Vanillin Hydrodeoxygenation Catalyst. ACS Appl. Mater. Interfaces 2019, 11, 24140–24153. [Google Scholar] [CrossRef]
- Zheng, Z.-H.; Chaemchuen, S.; Gu, J.-F.; Hang, J.; Sang, W.; Wang, J.-C.; Yuan, Y.; Chen, C. Nanostructured bimetallic Zn/Co in N-doped carbon as an efficient catalyst for the alcohol dehydrogenation to carboxylic acids under solvent-free conditions. J. Mater. Sci. Technol. 2023, 161, 111–122. [Google Scholar] [CrossRef]
- Gu, J.-F.; Chen, C.; Zheng, Z.-H.; Hang, J.; Sang, W.; Wang, J.-C.; Yuan, Y.; Chaemchuen, S.; Verpoort, F. Zeolitic imidazolate framework-8 as an efficient and facile heterogeneous catalyst for the acceptorless alcohol dehydrogenation to carboxylates. J. Catal. 2023, 417, 202–212. [Google Scholar] [CrossRef]
- Chen, C.; Hang, J.; Wang, Z.-Q.; Zheng, Z.-H.; Gu, J.-F.; Sang, W.; Yuan, Y.; Chaemchuen, S.; Verpoort, F. Rational design of cobalt catalysts embedded in N-Doped carbon for the alcohol dehydrogenation to carboxylic acids. Mol. Catal. 2023, 535, 112891. [Google Scholar] [CrossRef]
- Gunanathan, C.; Milstein, D. Applications of Acceptorless Dehydrogenation and Related Transformations in Chemical Synthesis. Science 2013, 341, 1229712. [Google Scholar] [CrossRef]
- Sawama, Y.; Morita, K.; Yamada, T.; Nagata, S.; Yabe, Y.; Monguchi, Y.; Sajiki, H. Rhodium-on-carbon catalyzed hydrogen scavenger- and oxidant-free dehydrogenation of alcohols in aqueous media. Green Chem. 2014, 16, 3439–3443. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, Y.; Liu, Y.; Wang, Q.; Fu, X.; Liu, Q. A general and efficient Mn-catalyzed acceptorless dehydrogenative coupling of alcohols with hydroxides into carboxylates. Org. Chem. Front. 2018, 5, 1248–1256. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Tang, X.S.; Yang, Z.Q.; Yu, B.Y.; Wang, H.J.; Sang, W.; Yuan, Y.; Chen, C.; Verpoort, F. Highly active bidentate N-heterocyclic carbene/ruthenium complexes performing dehydrogenative coupling of alcohols and hydroxides in open air. Chem. Commun. 2019, 55, 8591–8594. [Google Scholar] [CrossRef]
- Koley, P.; Shit, S.C.; Joseph, B.; Pollastri, S.; Sabri, Y.M.; Mayes, E.L.H.; Nakka, L.; Tardio, J.; Mondal, J. Leveraging Cu/CuFe2O4-Catalyzed Biomass-Derived Furfural Hydrodeoxygenation: A Nanoscale Metal-Organic-Framework Template Is the Prime Key. ACS Appl. Mater. Interfaces 2020, 12, 21682–21700. [Google Scholar] [CrossRef]
- Pradhan, D.R.; Pattanaik, S.; Kishore, J.; Gunanathan, C. Cobalt-Catalyzed Acceptorless Dehydrogenation of Alcohols to Carboxylate Salts and Hydrogen. Org. Lett. 2020, 22, 1852–1857. [Google Scholar] [CrossRef] [PubMed]
- Toyooka, G.; Fujita, K.-I. Synthesis of Dicarboxylic Acids from Aqueous Solutions of Diols with Hydrogen Evolution Catalyzed by an Iridium Complex. ChemSusChem 2020, 13, 3820–3824. [Google Scholar] [CrossRef]
- Boro, B.; Koley, P.; Tan, H.L.; Biswas, S.; Paul, R.; Bhargava, S.; Liu, W.; Wong, B.M.; Mondal, J. Influence of the Intrinsic Nanocore Environment in a Pd-Metalated Porous Organic Polymer for Catalytic Biomass-Derived Furfural Upgrading. ACS Appl. Nano Mater. 2022, 5, 14706–14721. [Google Scholar] [CrossRef]
- Paul, R.; Sarkar, C.; Jain, M.; Xu, S.; Borah, K.; Duy Quang, D.; Pao, C.-W.; Bhattacharya, S.; Mondal, J. Ferrocene-derived Fe-metalated porous organic polymer for the core planarity-triggered detoxification of chemical warfare agents. Chem. Commun. 2022, 58, 7789–7792. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, Z.-Q.; Cheng, H.; Zheng, Z.-H.; Yuan, Y.; Chen, C.; Verpoort, F. Gram-scale synthesis of carboxylic acids via catalytic acceptorless dehydrogenative coupling of alcohols and hydroxides at an ultralow Ru loading. Appl. Catal. A 2022, 630, 118443. [Google Scholar] [CrossRef]
- Dahl, E.W.; Louis-Goff, T.; Szymczak, N.K. Second sphere ligand modifications enable a recyclable catalyst for oxidant-free alcohol oxidation to carboxylates. Chem. Commun. 2017, 53, 2287–2289. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Luo, Q.; Meng, X.; Li, R.; Zhang, J.; Peng, T. Ru(II) complexes bearing 2,6-bis(benzimidazole-2-yl)pyridine ligands: A new class of catalysts for efficient dehydrogenation of primary alcohols to carboxylic acids and H2 in the alcohol/CsOH system. J. Organomet. Chem. 2017, 830, 11–18. [Google Scholar] [CrossRef]
- Sarbajna, A.; Dutta, I.; Daw, P.; Dinda, S.; Rahaman, S.M.W.; Sarkar, A.; Bera, J.K. Catalytic Conversion of Alcohols to Carboxylic Acid Salts and Hydrogen with Alkaline Water. ACS Catal. 2017, 7, 2786–2790. [Google Scholar] [CrossRef]
- Singh, A.; Singh, S.K.; Saini, A.K.; Mobin, S.M.; Mathur, P. Facile oxidation of alcohols to carboxylic acids in basic water medium by employing ruthenium picolinate cluster as an efficient catalyst. Appl. Organomet. Chem. 2018, 32, e4574. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Singh, S.K. Ruthenium Catalyzed Dehydrogenation of Alcohols and Mechanistic Study. Inorg. Chem. 2019, 58, 14912–14923. [Google Scholar] [CrossRef]
- Casas, F.; Trincado, M.; Rodriguez-Lugo, R.; Baneerje, D.; Grützmacher, H. A Diaminopropane Diolefin Ru(0) Complex Catalyzes Hydrogenation and Dehydrogenation Reactions. ChemCatChem 2019, 11, 5241–5251. [Google Scholar] [CrossRef]
- Gong, D.; Hu, B.; Chen, D. Bidentate Ru(ii)-NC complexes as catalysts for the dehydrogenative reaction from primary alcohols to carboxylic acids. Dalton Trans. 2019, 48, 8826–8834. [Google Scholar] [CrossRef]
- Liu, H.M.; Jian, L.; Li, C.; Zhang, C.C.; Fu, H.Y.; Zheng, X.L.; Chen, H.; Li, R.X. Dehydrogenation of Alcohols to Carboxylic Acid Catalyzed by in Situ-Generated Facial Ruthenium-CPP Complex. J. Org. Chem. 2019, 84, 9151–9160. [Google Scholar] [CrossRef]
- Ania, C.O.; Garcia-Perez, E.; Haro, M.; Gutierrez-Sevillano, J.J.; Valdes-Solis, T.; Parra, J.B.; Calero, S. Understanding Gas-Induced Structural Deformation of ZIF-8. J. Phys. Chem. Lett. 2012, 3, 1159–1164. [Google Scholar] [CrossRef]
- Yin, H.; Kim, H.; Choi, J.; Yip, A.C.K. Thermal stability of ZIF-8 under oxidative and inert environments: A practical perspective on using ZIF-8 as a catalyst support. Chem. Eng. J. 2015, 278, 293–300. [Google Scholar] [CrossRef]
- Wu, P.; Lu, G.; Cai, C. Cobalt-molybdenum synergistic catalysis for the hydrogenolysis of terephthalate-based polyesters. Green Chem. 2021, 23, 8666–8672. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, F.; Yu, J.; Zhang, X.; Lu, G.-P. Iron single-atom anchored N-doped carbon as a ‘laccase-like’ nanozyme for the degradation and detection of phenolic pollutants and adrenaline. J. Hazard. Mater. 2022, 425, 122763. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, P.; Gao, W.; Ma, S.; Zhang, G.; Cao, R.; Cho, J.; Wang, H.-L.; Wu, G. Graphene/Graphene-Tube Nanocomposites Templated from Cage-Containing Metal-Organic Frameworks for Oxygen Reduction in Li-O-2 Batteries. Adv. Mater. 2014, 26, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Chaemchuen, S.; Xiao, X.; Ghadamyari, M.; Mousavi, B.; Klomkliang, N.; Yuan, Y.; Verpoort, F. Robust and efficient catalyst derived from bimetallic Zn/Co zeolitic imidazolate frameworks for CO2 conversion. J. Catal. 2019, 370, 38–45. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Hu, M.; Wang, H.; Huang, H.-S.; Fujita, T.; Wu, K.C.W.; Chen, L.-C.; Yamauchi, Y.; Ariga, K. Nanoporous carbons through direct carbonization of a zeolitic imidazolate framework for supercapacitor electrodes. Chem. Commun. 2012, 48, 7259–7261. [Google Scholar] [CrossRef]
- Dabiri, M.; Salmasi, A.M.; Salarinejad, N.; Movahed, S.K. Decoration of ruthenium nanoparticles on nitrogen and phosphorus-doped carbon as a robust catalyst for the reduction of nitroarenes. J. Mol. Struct. 2023, 1287, 135609. [Google Scholar] [CrossRef]
- Arechederra, R.L.; Artyushkova, K.; Atanassov, P.; Minteer, S.D. Growth of Phthalocyanine Doped and Undoped Nanotubes Using Mild Synthesis Conditions for Development of Novel Oxygen Reduction Catalysts. ACS Appl. Mater. Interfaces 2010, 2, 3295–3302. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Shang, N.; Gao, S.; Gao, Y.; Wang, C. Ultra dispersed cobalt anchored on nitrogen-doping ordered porous carbon as an efficient transfer hydrogenation catalyst. Appl. Surf. Sci. 2019, 491, 544–552. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Sun, H.; Wang, H.; Rong, F.; He, L.; Lou, Y.; Zhang, S.; Zhang, Z.; Du, M. CoOx/CoNy nanoparticles encapsulated carbon-nitride nanosheets as an efficiently trifunctional electrocatalyst for overall water splitting and Zn-air battery. Appl. Catal. B 2020, 279, 119407. [Google Scholar] [CrossRef]
- Cheng, T.; Yu, H.; Peng, F.; Wang, H.; Zhang, B.; Su, D. Identifying active sites of CoNC/CNT from pyrolysis of molecularly defined complexes for oxidative esterification and hydrogenation reactions. Catal. Sci. Technol. 2016, 6, 1007–1015. [Google Scholar] [CrossRef]










| Entry | Catalyst | Solvent | y | n | Conversion (%) b | Yield (%) b |
|---|---|---|---|---|---|---|
| 1 a | Ru0.05@Co-NC | toluene | 0.5 | 16 | 51 | 50 |
| 2 a | Ru0.1@Co-NC | toluene | 0.5 | 16 | 49 | 48 |
| 3 a | Ru0.15@Co-NC | toluene | 0.5 | 16 | 53 | 49 |
| 4 a | Ru0.2@Co-NC | toluene | 0.5 | 16 | 50 | 47 |
| 5 a | Ru0.05@Co-NC | toluene | 0.5 | 24 | 68 | 65 |
| 6 c | Ru0.05@Co-NC | toluene | 0.5 | 24 | 86 | 85(83 d) |
| 7 a | Ru0.05@Co-NC | 1,4-dioxane | 0.5 | 24 | 73 | 68 |
| 8 a | Ru0.05@Co-NC | m-xylene | 0.5 | 24 | 82 | 78 |
| 9 a | Ru0.05@Co-NC | water | 0.5 | 24 | 15 | 9 |
| 10 a | Ru0.05@Co-NC | - | 0.5 | 24 | 30 | 28 |
| 11 c | Ru0.05@Co-NC | toluene | 0.25 | 24 | 74 | 72 |
| 12 c | Ru0.05@Co-NC | toluene | 0.75 | 24 | 77 | 75 |
| 13 c,e | Ru0.05@Co-NC | toluene | 0.5 | 24 | 85 | 84 |
| 14 c,e | Ru0.05@Co-NC | toluene | 0.5 | 24 | 89 | 86 |
| 15 c,f | Co-NC | toluene | 0.5 | 24 | 51 | 48 |
| 16 c | - | toluene | 0.5 | 24 | 6 | 5 |
| 17 g | Ru0.05@Co-NC | toluene | 0.5 | 24 | 4 | <1 |

| Entry a | Catalyst | Conversion (%) b | Yield (%) b |
|---|---|---|---|
| 1 | Ru0.05@Co-NC | 86 | 85 |
| 2 | Ru0.05@Co-NC-HCl | 84 | 82 |
| 3 | Ru0.05@Co-NC-HNO3 | 54 | 51 |
| 4 | Co-NC | 51 | 48 |
| 5 | Co-NC-HCl | 54 | 50 |
| 6 | Co-NC-HNO3 | 22 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Hang, J.; Zhang, S.; Yuan, Y.; Verpoort, F.; Chen, C. Zeolitic Imidazolium Frameworks-Derived Ru-Based Composite Materials Enable the Catalytic Dehydrogenation of Alcohols to Carboxylic Acids. Catalysts 2023, 13, 1225. https://doi.org/10.3390/catal13081225
Chen Z, Hang J, Zhang S, Yuan Y, Verpoort F, Chen C. Zeolitic Imidazolium Frameworks-Derived Ru-Based Composite Materials Enable the Catalytic Dehydrogenation of Alcohols to Carboxylic Acids. Catalysts. 2023; 13(8):1225. https://doi.org/10.3390/catal13081225
Chicago/Turabian StyleChen, Zhan, Jing Hang, Song Zhang, Ye Yuan, Francis Verpoort, and Cheng Chen. 2023. "Zeolitic Imidazolium Frameworks-Derived Ru-Based Composite Materials Enable the Catalytic Dehydrogenation of Alcohols to Carboxylic Acids" Catalysts 13, no. 8: 1225. https://doi.org/10.3390/catal13081225
APA StyleChen, Z., Hang, J., Zhang, S., Yuan, Y., Verpoort, F., & Chen, C. (2023). Zeolitic Imidazolium Frameworks-Derived Ru-Based Composite Materials Enable the Catalytic Dehydrogenation of Alcohols to Carboxylic Acids. Catalysts, 13(8), 1225. https://doi.org/10.3390/catal13081225