Catalytic Conversion of Cyclopentanone into Dimethyl Adipate over Solid Basic Catalysts with Dimethyl Carbonate
Abstract
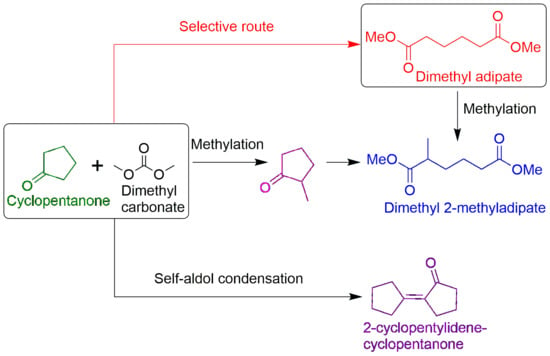
:1. Introduction
2. Results and Discussion
2.1. A Kinetic Study of the Reaction Using MgO and MgCO3 (HC)
2.2. Study of the Selective Pathway (Route a) Using MgO and MgCO3 (HC) Catalysts
2.3. Cyclopentanone Methylation (Route b) Using KOCH3 Catalyst
2.4. The Self-Aldol Condensation of CPO (Route c) Using MgCO3 (HC), MgO and Ca(OCH3)2 Catalysts
2.5. DAP Productivity for Different Basic Solid Catalysts
3. Materials and Methods
3.1. Reagents and Catalysts
3.2. Catalytic Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Acronyms
| 2-Carbomethoxy-cyclopentanone | CMCP |
| 2-Cyclopentylidene-cyclopentanone | CPCP |
| 2-Methyl cyclopentanone | MCP |
| Adipic acid | AdA |
| Cyclopentanone | CPO |
| Dimethyl 2-methyladipate | DMAP |
| Dimethyl adipate | DAP |
| Dimethyl carbonate | DMC |
References
- Castellan, A.; Bart, J.C.J.; Cavallaro, S. Industrial Production and Use of Adipic Acid. Catal. Today 1991, 9, 237–254. [Google Scholar] [CrossRef]
- Han, J. A Bio-Based ‘Green’ Process for Catalytic Adipic Acid Production from Lignocellulosic Biomass Using Cellulose and Hemicellulose Derived γ-Valerolactone. Energy Convers. Manag. 2016, 129, 75–80. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Cavallaro, S. Transiting from Adipic Acid to Bioadipic Acid. 1, Petroleum-Based Processes. Ind. Eng. Chem. Res. 2015, 54, 1–46. [Google Scholar] [CrossRef]
- Shimizu, A.; Tanaka, K.; Fujimori, M. Abatement Technologies for N2O Emissions in the Adipic Acid Industry. Chemosph.-Glob. Chang. Sci. 2000, 2, 425–434. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Cavallaro, S. Transiting from Adipic Acid to Bioadipic Acid. Part II. Biosynthetic Pathways. Ind. Eng. Chem. Res. 2015, 54, 567–576. [Google Scholar] [CrossRef]
- Iglesias, J.; Martínez-Salazar, I.; Maireles-Torres, P.; Martin Alonso, D.; Mariscal, R.; López Granados, M. Advances in Catalytic Routes for the Production of Carboxylic Acids from Biomass: A Step Forward for Sustainable Polymers. Chem. Soc. Rev. 2020, 49, 5704–5771. [Google Scholar] [CrossRef] [PubMed]
- Gallezot, P. Conversion of Biomass to Selected Chemical Products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- El Tawil-Lucas, M.; Montaña, M.; Macias-Villasevil, M.; Moreno, J.; Iglesias, J. Isomerization of Hemicellulose Aldoses to Ketoses Catalyzed by Basic Anion Resins: Catalyst Screening and Stability Studies. Catalysts 2023, 13, 1301. [Google Scholar] [CrossRef]
- Alba-Rubio, A.C.; Cecilia, J.A.; Jiménez-Gómez, C.P.; García-Sancho, C.; Cassidy, A.; Moreno-Tost, R.; Maireles-Torres, P. The Role of MnOx in Cu-MnOx/SiO2 Catalysts for the Gas-Phase Hydrogenation of Furfural. Mol. Catal. 2023, 546, 113224. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtarová, K. Selective Transformation of Furfural to Cyclopentanone. Catal. Commun. 2012, 24, 100–104. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Katta, L.; Thrimurthulu, G.; Reddy, B.M. Vapor Phase Synthesis of Cyclopentanone over Nanostructured Ceria-Zirconia Solid Solution Catalysts. J. Ind. Eng. Chem. 2013, 19, 1517–1524. [Google Scholar] [CrossRef]
- Orozco-Saumell, A.; Mariscal, R.; Vila, F.; López-Granados, M.; Martín Alonso, D. Hydrogenation of Furfural to Cyclopentanone in Tert—Butanol-Water Medium: A Study of the Reaction Intermediates Reactivity Using Cu/ZnO/Al2O3 as Catalyst. Catalysts 2023, 13, 1394. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How Catalysts and Experimental Conditions Determine the Selective Hydroconversion of Furfural and 5-Hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A Renewable and Versatile Platform Molecule for the Synthesis of Chemicals and Fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- López Granados, M. Cyclopentanone and Its Derived Biofuels. In Furfural: An Entry Point of Lignocellulose in Biorefineries to Produce Renewable Chemicals, Polymers, and Biofuels; López Granados, M., Martín Alonso, D., Eds.; World Scientific Publishing Co. Pte. Ltd: Singapore, 2018; pp. 157–168. [Google Scholar]
- Tundo, P.; Selva, M. The Chemistry of Dimethyl Carbonate. Acc. Chem. Res. 2002, 35, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Tundo, P. New Developments in Dimethyl Carbonate Chemistry. Pure Appl. Chem. 2001, 73, 1117–1124. [Google Scholar] [CrossRef]
- Tamboli, A.H.; Chaugule, A.A.; Kim, H. Catalytic Developments in the Direct Dimethyl Carbonate Synthesis from Carbon Dioxide and Methanol. Chem. Eng. J. 2017, 323, 530–544. [Google Scholar] [CrossRef]
- Hamed, O.; El-Qisairi, A.; Henry, P.M. Palladium(II) Catalyzed Carbonylation of Ketones. Tetrahedron Lett. 2000, 41, 3021–3024. [Google Scholar] [CrossRef]
- Hamed, O.; El-Qisairi, A.; Henry, P.M. Palladium(II)-Catalyzed Oxidation of Aldehydes and Ketones. 1. Carbonylation of Ketones with Carbon Monoxide Catalyzed by Palladium(II) Chloride in Methanol. J. Org. Chem. 2001, 66, 180–185. [Google Scholar] [CrossRef]
- Hattori, H. Solid Base Catalysts: Fundamentals and Their Applications in Organic Reactions. Appl. Catal. A Gen. 2015, 504, 103–109. [Google Scholar] [CrossRef]
- Selva, M.; Marques, C.A.; Tundo, P. The Addition Reaction of Dialkyl Carbonates to Ketones. Gazz. Chim. Ital. 1993, 123, 515–518. [Google Scholar]
- Tundo, P.; Memoli, S.; Selva, M. Synthesis of α,ω-Diesters. WO Patent 0,214,257, 21 February 2002. [Google Scholar]
- Zhang, G.S.; Zhu, M.M.; Zhang, Q.; Liu, Y.M.; He, H.Y.; Cao, Y. Towards Quantitative and Scalable Transformation of Furfural to Cyclopentanone with Supported Gold Catalysts. Green Chem. 2016, 18, 2155–2164. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Z.; Jia, Z.; Shuai, L. Synthesis of Dimethyl Adipate from Cyclopentanone and Dimethyl Carbonate over Solid Base Catalysts. Sci. China Chem. 2012, 55, 380–385. [Google Scholar] [CrossRef]
- Annese, C.; D’Accolti, L.; Fusco, C.; Tommasi, I. Reactivity of 1,3-Dimethylimidazolium-2-Carboxylate with Dimethylcarbonate at High Temperature: Unexpected 2-Ethyl-Functionalisation of the Imidazolium Moiety and Employment of the NHC-CO2/Dimethylcarbonate System in a Base Promoted Reaction. Catal. Commun. 2014, 46, 94–97. [Google Scholar] [CrossRef]
- Clayden, J.; Greeves, N.; Warren, S. The Chemistry of Carbanions. IX. The Potassium and Lithium Enolates Derived from Cyclic Ketones. J. Org. Chem. 1965, 30, 1341–1348. [Google Scholar]
- House, H.O.; Czuba, L.J.; Gall, M.; Olmstead, H.D. The Chemistry of Carbanions. XVIII. Preparation of Trimethylsilyl Enol Ethers. J. Org. Chem. 1968, 34, 2324–2336. [Google Scholar] [CrossRef]
- Waddell, D.C.; Thiel, I.; Clark, T.D.; Marcum, S.T.; Mack, J. Making Kinetic and Thermodynamic Enolates via Solvent-Free High Speed Ball Milling. Green Chem. 2010, 12, 209–221. [Google Scholar] [CrossRef]
- Li, G.; Dissanayake, S.; Suib, S.L.; Resasco, D.E. Activity and Stability of Mesoporous CeO2 and ZrO2 Catalysts for the Self-Condensation of Cyclopentanone. Appl. Catal. B Environ. 2020, 267, 118373. [Google Scholar] [CrossRef]
- Lange, J.P. Catalysis for Biorefineries-Performance Criteria for Industrial Operation. Catal. Sci. Technol. 2016, 6, 4759–4767. [Google Scholar] [CrossRef]







| # | Catalyst | Substrate | Catalytic Results (%) | ||||
|---|---|---|---|---|---|---|---|
| XSubs | YDAP Route a | YMCP Route b | YDMAP Route b/Further a | YCPCP Route c | |||
| 1 | MgCO3 (HC) | CPO a | 85 | 29 | 0 | 3 | 10 |
| 2 | MgCO3 (HC) | MCP a | 80 | 0 | - | 30 | 0 |
| 3 | MgCO3 (HC) | DAP b | 32 | - | - | 5 | - |
| 4 | MgO | DAP b | 32 | - | - | 5 | - |
| # | Catalyst | CPO/DMC (wt%) | Cat/CPO (wt%) | T (K) | t (h) | Prod DAP (g/gcat·h) | Ref |
|---|---|---|---|---|---|---|---|
| 1 | Ca(OCH3)2 | 9.3 | 10 | 533 | 1.5 | 3.45 | This work |
| 2 | MgCO3 (HC) | 9.3 | 10 | 533 | 2 | 3.00 | This work |
| 3 | MgO | 9.3 | 10 | 533 | 2 | 2.90 | This work |
| 4 | MgO | 14.0 | 14.0 | 533 | 5 | 1.09 | [25] |
| 5 | MgO | 2.2 | 42.0 | 533 | 3 | 0.65 | [24] |
| 6 | CeO2-nanorod | 2.2 | 42.0 | 533 | 5 | 0.69 | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Salazar, I.; Orozco-Saumell, A.; López Granados, M.; Mariscal, R. Catalytic Conversion of Cyclopentanone into Dimethyl Adipate over Solid Basic Catalysts with Dimethyl Carbonate. Catalysts 2024, 14, 86. https://doi.org/10.3390/catal14010086
Martínez-Salazar I, Orozco-Saumell A, López Granados M, Mariscal R. Catalytic Conversion of Cyclopentanone into Dimethyl Adipate over Solid Basic Catalysts with Dimethyl Carbonate. Catalysts. 2024; 14(1):86. https://doi.org/10.3390/catal14010086
Chicago/Turabian StyleMartínez-Salazar, Irene, Ana Orozco-Saumell, Manuel López Granados, and Rafael Mariscal. 2024. "Catalytic Conversion of Cyclopentanone into Dimethyl Adipate over Solid Basic Catalysts with Dimethyl Carbonate" Catalysts 14, no. 1: 86. https://doi.org/10.3390/catal14010086
APA StyleMartínez-Salazar, I., Orozco-Saumell, A., López Granados, M., & Mariscal, R. (2024). Catalytic Conversion of Cyclopentanone into Dimethyl Adipate over Solid Basic Catalysts with Dimethyl Carbonate. Catalysts, 14(1), 86. https://doi.org/10.3390/catal14010086