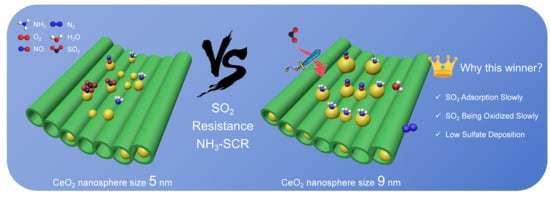
Influence of Particle Size of CeO2 Nanospheres Encapsulated in SBA-15 Mesopores on SO2 Tolerance during NH3-SCR Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pore Analysis of SBA-15
2.2. Particle Size Control of Nanospheres
2.3. NH3-SCR Performance and SO2 Resistance of the Catalyst
2.4. Material Structure and Morphology
2.4.1. Phase Analysis (XRD and FT-IR)
2.4.2. Morphology of Catalysts (TEM)
2.5. Chemisorption Test
2.5.1. Acid Site Distribution and Intensity (NH3-TPD)
2.5.2. Redox Site Distribution and Intensity (H2-TPR)
2.6. Valence Testing of Surface Atoms (XPS)
2.7. Sulfation Rate Test of Active Components
2.8. Reaction Kinetics
2.9. Thermal Decomposition of Sulfate (TGA)
2.10. Reaction Mechanism
2.10.1. NH3 Passed over Pre-Adsorbed NO + O2
2.10.2. NO + O2 Passed over Pre-Adsorbed NH3
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Performance Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, M.; Shang, F.; Lu, X.; Huang, X.; Song, Y.; Liu, B.; Zhang, Q.; Liu, X.; Cao, J.; Xu, T.; et al. Unexpected response of nitrogen deposition to nitrogen oxide controls and implications for land carbon sink. Nat. Commun. 2022, 13, 3126. [Google Scholar] [CrossRef]
- Wang, X.; Li, B.; Wang, Y.; Wei, T.; Li, S.; Li, W. Insight into the dynamic behaviors of reactants with temperature over a TiOx-based catalyst for NOx removal via NH3-SCR. Appl. Surf. Sci. 2022, 605, 154689. [Google Scholar] [CrossRef]
- An, X.; Feng, C.; Liu, J.; Cheng, G.; Du, Y.; Fan, Z.; Wu, X. Insight into the sulfur resistance of manganese oxide for NH3-SCR: Perspective from the valence state distributions. Appl. Surf. Sci. 2022, 592, 153223. [Google Scholar] [CrossRef]
- Zhao, W.; Rong, J.; Luo, W.; Long, L.; Yao, X. Enhancing the K-poisoning resistance of CeO2-SnO2 catalyst by hydrothermal method for NH3-SCR reaction. Appl. Surf. Sci. 2022, 579, 152176. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; van Dillen, A.J.; Geus, J.W.; Janssen, F.J.J.G. Selective catalytic reduction of NOx with NH3 over a V2O5/TiO2 on silica catalyst. Catal. Today 1988, 2, 569–579. [Google Scholar] [CrossRef]
- Martín-Martínez, J.M.; Singoredjo, L.; Mittelmeijer-Hazeleger, M.; Kapteijn, F.; Moulijn, J.A. Selective catalytic reduction of no with NH3 over activated carbons. I: Effect of origin and activation procedure on activity. Carbon 1994, 32, 897–904. [Google Scholar] [CrossRef]
- Kakuta, N.; Sugino, Y.; Rachi, H.; Ohkita, H.; Mizushima, T. Chemical removal of CeO2 segregated on the surface of CeO2-ZrO2 binary oxides for improvement of OSC. Top. Catal. 2009, 52, 1888. [Google Scholar] [CrossRef]
- Kakuta, N.; Morishima, N.; Kotobuki, M.; Iwase, T.; Mizushima, T.; Sato, Y.; Matsuura, S. Oxygen storage capacity (OSC) of aged Pt/CeO2/Al2O3 catalysts: Roles of Pt and CeO2 supported on Al2O3. Appl. Surf. Sci. 1997, 121–122, 408–412. [Google Scholar] [CrossRef]
- Han, X.; Liu, K.; Bian, M.; Fang, Y.; Sun, J.; Zhang, Y.; Yang, X. CeO2−δ nanoparticles supported on SnNb2O6 nanosheets for selective catalytic reduction of NOx with NH3. ACS Appl. Nano Mater. 2022, 5, 13529–13541. [Google Scholar] [CrossRef]
- Li, X.; He, E.; Zhang, M.; Peijnenburg, W.J.G.M.; Liu, Y.; Song, L.; Cao, X.; Zhao, L.; Qiu, H. Interactions of CeO2 nanoparticles with natural colloids and electrolytes impact their aggregation kinetics and colloidal stability. J. Hazard. Mater. 2020, 386, 121973. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zhang, Z.R.; Suo, J.S.; Ben, L.S. Synthesis of mesoporous silica molecular sieves via a novel templating scheme. Chin. Chem. Lett. 1999, 129, 23–30. [Google Scholar]
- Shen, J.; Hess, C. Controlling the dispersion of ceria using nanoconfinement: Application to CeO2/SBA-15 catalysts for NH3-SCR. Mater. Adv. 2021, 2, 7400–7412. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Cao, Y.; Yao, X.; Ge, C.; Gao, F.; Deng, Y.; Tang, C.; Dong, L. Getting insight into the influence of SO2 on TiO2/CeO2 for the selective catalytic reduction of NO by NH3. Appl. Catal. B-Environ. 2015, 165, 589–598. [Google Scholar] [CrossRef]
- Zhu, J.; Ciolca, D.; Liu, L.; Parastaev, A.; Kosinov, N.; Hensen, E.J.M. Flame synthesis of Cu/ZnO-CeO2 catalysts: Synergistic metal–support interactions promote CH3OH selectivity in CO2 hydrogenation. ACS Catal. 2021, 11, 4880–4892. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Guo, K.; Li, L.; Zou, W.; Dong, L. Cavity size dependent SO2 resistance for NH3-SCR of hollow structured CeO2-TiO2 catalysts. Catal. Commun. 2019, 128, 105719. [Google Scholar] [CrossRef]
- Hu, X.; Chen, J.; Qu, W.; Liu, R.; Tang, X. Sulfur-resistant ceria-based low-temperature SCR catalysts with the non-bulk electronic states of ceria. Environ. Sci. Technol. 2021, 55, 5435–5441. [Google Scholar] [CrossRef]
- Li, L.L.; Sun, B.W.; Sun, J.F.; Yu, S.H.; Ge, C.Y.; Tang, C.J.; Dong, L. Novel MnOx-CeO2 nanosphere catalyst for low-temperature NH3-SCR. Catal. Commun. 2017, 100, 98–102. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Hu, P.; Ma, S.; Li, Y. The effects of PVP surfactant in the direct and indirect hydrothermal synthesis processes of ceria nanostructures. Ceram. Int. 2016, 42, 18516–18520. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, X.; Xu, H.; Yuan, C. CeO2 spherical crystallites: Synthesis, formation mechanism, size control, and electrochemical property study. J. Phys. Chem. C 2007, 111, 1651–1657. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W.; Zhang, B.; Li, H.; Zhang, Q. Controllable synthesis of spherical cerium oxide particles. RSC Adv. 2016, 6, 30956–30962. [Google Scholar] [CrossRef]
- Xie, R.; Ma, L.; Li, Z.; Qu, Z.; Yan, N.; Li, J. Review of sulfur promotion effects on metal oxide catalysts for NOx emission control. ACS Catal. 2021, 11, 13119–13139. [Google Scholar] [CrossRef]
- Guo, K.; Ji, J.W.; Song, W.; Sun, J.F.; Tang, C.J.; Dong, L. Conquering ammonium bisulfate poison over low-temperature NH3-SCR catalysts: A critical review. Appl. Catal. B-Environ. 2021, 297, 120388. [Google Scholar] [CrossRef]
- Muzio, L.; Bogseth, S.; Himes, R.; Chien, Y.C.; Dunn-Rankin, D. Ammonium bisulfate formation and reduced load SCR operation. Fuel 2017, 206, 180–189. [Google Scholar] [CrossRef]
- Zhang, W.J.; Liu, G.F.; Jiang, J.; Tan, Y.C.; Wang, Q.; Gong, C.H.; Shen, D.K.; Wu, C.F. Temperature sensitivity of the selective catalytic reduction (SCR) performance of Ce-TiO2 in the presence of SO2. Chemosphere 2020, 243, 125419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, Y.; Dai, H.; He, H.; Au, C.T. A comparative investigation on the properties of Cr-SBA-15 and CrOx/SBA-15. Catal. Today 2008, 131, 42–54. [Google Scholar] [CrossRef]
- Bae, Y.K.; Kim, T.-W.; Kim, J.-R.; Kim, Y.; Ha, K.-S.; Chae, H.-J. Enhanced SO2 tolerance of V2O5-Sb2O3/TiO2 catalyst for NO reduction with co-use of ammonia and liquid ammonium nitrate. J. Ind. Eng. Chem. 2021, 96, 277–283. [Google Scholar] [CrossRef]
- Han, X.; Bian, M.; Liu, K.; Yang, X.; Zheng, D.; Yang, X.; Zhang, Y. Excellent MCM-49 Supported CeCuOx nanocatalyst with ultrawide operating temperature window and strong anti-alkali ability for NH3-SCR. ChemNanoMat 2023, 9, e202300407. [Google Scholar] [CrossRef]
- Han, X.; Jin, S.; Rao, C.; Fang, Y.; Hu, B.; Liu, K.; Zhang, Y.; Yang, X. Mo-modified Pt/CeO2 nanorods with high propane catalytic combustion activity: Comparison of phosphoric, sulphuric and molybdic acid modifications. Mol. Catal. 2023, 548, 113426. [Google Scholar] [CrossRef]
- Xie, S.; Tan, W.; Li, Y.; Ma, L.; Ehrlich, S.N.; Deng, J.; Xu, P.; Gao, F.; Dong, L.; Liu, F. Copper single atom-triggered niobia–ceria catalyst for efficient low-temperature reduction of nitrogen oxides. ACS Catal. 2022, 12, 2441–2453. [Google Scholar] [CrossRef]
- Maqbool, M.S.; Pullur, A.K.; Ha, H.P. Novel sulfation effect on low-temperature activity enhancement of CeO2-added Sb-V2O5/TiO2 catalyst for NH3-SCR. Appl. Catal. B-Environ. 2014, 152, 28–37. [Google Scholar] [CrossRef]
- Peng, Y.; Li, J.; Si, W.; Li, X.; Shi, W.; Luo, J.; Fu, J.; Crittenden, J.; Hao, J. Ceria promotion on the potassium resistance of MnOx/TiO2 SCR catalysts: An experimental and DFT study. Chem. Eng. J. 2015, 269, 44–50. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Y.; Liu, S.; Zheng, C.; Gao, X.; Nova, I.; Tronconi, E. Improvement in activity and alkali resistance of a novel V-Ce(SO4)2/Ti catalyst for selective catalytic reduction of NO with NH3. Appl. Catal. B-Environ. 2017, 206, 449–460. [Google Scholar] [CrossRef]
- Wang, X.; Cong, Q.; Chen, L.; Shi, Y.; Shi, Y.; Li, S.; Li, W. The alkali resistance of CuNbTi catalyst for selective reduction of NO by NH3: A comparative investigation with VWTi catalyst. Appl. Catal. B-Environ. 2019, 246, 166–179. [Google Scholar] [CrossRef]
- Bian, M.; Liu, K.; Han, X.; Fang, Y.; Liu, Q.; Zhang, Y.; Yang, X. Novel manganese-based assembled nanocatalyst with “nitrous oxide filter” for efficient NH3-SCR in wide low-temperature window: Optimization, design and mechanism. Fuel 2023, 331, 125857. [Google Scholar] [CrossRef]
- Liu, J.; Shi, X.; Shan, Y.; Yan, Z.; He, H. Hydrothermal stability of CeO2-WO3-ZrO2 mixed oxides for selective catalytic reduction of NOx by NH3. Environ. Sci. Technol. 2018, 52, 11769–11777. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Yang, R.T. Characterization and FT-IR studies of MnOx-CeO2 catalyst for low-temperature SCR of NO with NH3. J. Phys. Chem. B 2004, 108, 4463–4469. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.L.; Ge, C.Y.; Li, T.Z.; Li, C.J.; Li, S.X.; Xiong, F.; Dong, L. Promoting N2 selectivity of CeMnOx catalyst by supporting TiO2 in NH3-SCR reaction. Ind. Eng. Chem. Res. 2019, 58, 6325–6332. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I. Identification of neutral and charged NxOy surface species by IR spectroscopy. Catal. Rev. 2000, 42, 71–144. [Google Scholar] [CrossRef]











| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| SBA-15 | 496 | 1.20 | 10.07 |
| Catalyst | Ce3+/(Ce3+ + Ce4+) | Ce Atoms on Surface (Atomic Percentage) | S Atoms on Surface (Atomic Percentage) |
|---|---|---|---|
| 5n-Ce/SBA | 22.80% | 5.79% | — |
| 6n-Ce/SBA | 26.00% | 2.52% | |
| 7n-Ce/SBA | 21.43% | 2.68% | |
| 9n-Ce/SBA | 27.70% | 2.41% | |
| 5n-Ce/SBA-S | 24.01% | 4.46% | 1.28% |
| 6n-Ce/SBA-S | 23.98% | 4.64% | 1.79% |
| 7n-Ce/SBA-S | 25.72% | 1.58% | 5.63% |
| 9n-Ce/SBA-S | 23.57% | 5.36% | 3.05% |
| Catalyst | Reaction Order of | Rate Equation | ||
|---|---|---|---|---|
| NO | NH3 | O2 | ||
| 5n-Ce/SBA | 0.604 | 0.00041 | 0.138 | |
| 6n-Ce/SBA | 0.631 | 0.00050 | 0.112 | |
| 7n-Ce/SBA | 0.818 | 0.00045 | 0.099 | |
| 9n-Ce/SBA | 0.717 | 0.00032 | 0.115 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Bian, M.; Liu, K.; Yang, X.; Zheng, D.; Yang, X.; Zhang, Y. Influence of Particle Size of CeO2 Nanospheres Encapsulated in SBA-15 Mesopores on SO2 Tolerance during NH3-SCR Reaction. Catalysts 2024, 14, 151. https://doi.org/10.3390/catal14020151
Han X, Bian M, Liu K, Yang X, Zheng D, Yang X, Zhang Y. Influence of Particle Size of CeO2 Nanospheres Encapsulated in SBA-15 Mesopores on SO2 Tolerance during NH3-SCR Reaction. Catalysts. 2024; 14(2):151. https://doi.org/10.3390/catal14020151
Chicago/Turabian StyleHan, Xinyu, Mengyao Bian, Kaijie Liu, Xin Yang, Daying Zheng, Xiangguang Yang, and Yibo Zhang. 2024. "Influence of Particle Size of CeO2 Nanospheres Encapsulated in SBA-15 Mesopores on SO2 Tolerance during NH3-SCR Reaction" Catalysts 14, no. 2: 151. https://doi.org/10.3390/catal14020151
APA StyleHan, X., Bian, M., Liu, K., Yang, X., Zheng, D., Yang, X., & Zhang, Y. (2024). Influence of Particle Size of CeO2 Nanospheres Encapsulated in SBA-15 Mesopores on SO2 Tolerance during NH3-SCR Reaction. Catalysts, 14(2), 151. https://doi.org/10.3390/catal14020151