Recent Developments in Two-Dimensional Carbon-Based Nanomaterials for Electrochemical Water Oxidation: A Mini Review
Abstract
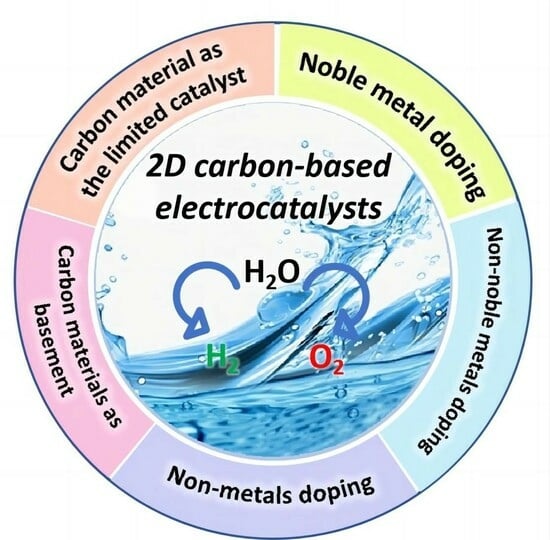
:1. Introduction
2. Precious Metal−Doped 2D Carbon-Based Electrocatalysts

3. Non−Precious Metal−Doped 2D Carbon−Based Electrocatalysts
4. Non−Metallic 2D Carbon−Based Electrocatalysts

5. 2D Carbon−Based Confined Electrocatalysts

6. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| HER | hydrogen evolution reaction |
| OER | oxygen evolution reaction |
| 2D | two−dimensional |
| VCNs | vertically aligned carbon nanosheets |
| Ru | ruthenium |
| ZIF-derived | zeolitic imidazolate frameworks derivatives |
| HMT | hexamethylenetetramine |
| GO | graphene oxide |
| Ir | iridium |
| N/B | bi−nonmetal |
| DFT | density functional theory |
| GDY | graphdiyne |
| CPT | cathodic polarization treatment |
| CVD | chemical vapor deposition |
| ABPBI | 2,5−benzimidazole |
| IPA | isopropyl alcohol |
| MOFs | metal−organic frameworks |
| C60 | buckminsterfullerene |
| h-BN | hexagonal boron nitride |
| BCN NSs | boron carbon nitride nanosheets |
| NG | N−doped graphene |
| EBP | exfoliated black phosphorus |
| R-graphyne | rectangular graphyne |
| HWE | hybrid water electrolysis |
References
- Wang, W.; Meng, J.; Hu, Y.; Wang, J.; Li, Q.; Yang, J. Thgraphene: A novel two-dimensional carbon allotrope as a potential multifunctional material for electrochemical water splitting and potassium-ion batteries. J. Mater. Chem. A 2022, 10, 9848–9857. [Google Scholar] [CrossRef]
- Shan, X.; Liu, J.; Mu, H.; Xiao, Y.; Mei, B.; Liu, W.; Lin, G.; Jiang, Z.; Wen, L.; Jiang, L. An Engineered Superhydrophilic/Superaerophobic Electrocatalyst Composed of the Supported CoMoSx Chalcogel for Overall Water Splitting. Angew. Chem. Int. Ed. 2019, 59, 1659–1665. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen society: From present to future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Khalafallah, D.; Zhang, Y.; Wang, H.; Lee, J.-M.; Zhang, Q. Energy-saving electrochemical hydrogen production via co-generative strategies in hybrid water electrolysis: Recent advances and perspectives. Chin. J. Catal. 2023, 55, 44–115. [Google Scholar] [CrossRef]
- Khalafallah, D.; Qiao, F.; Liu, C.; Wang, J.; Zhang, Y.; Wang, J.; Zhang, Q.; Notten, P.H.L. Heterostructured transition metal chalcogenides with strategic heterointerfaces for electrochemical energy conversion/Storage. Coord. Chem. Rev. 2023, 496, 215405. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Lewis, N.S. Research opportunities to advance solar energy utilization. Science 2016, 351, aad1920. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative Strategies for Electrocatalytic Water Splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, B.; Kim, H.; Kang, K. Recent Progress on Multimetal Oxide Catalysts for the Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1702774. [Google Scholar] [CrossRef]
- Sultan, S.; Tiwari, J.N.; Singh, A.N.; Zhumagali, S.; Ha, M.; Myung, C.W.; Thangavel, P.; Kim, K.S. Single Atoms and Clusters Based Nanomaterials for Hydrogen Evolution, Oxygen Evolution Reactions, and Full Water Splitting. Adv. Energy Mater. 2019, 9, 1900624. [Google Scholar] [CrossRef]
- Huang, B.; Liu, Y.; Xie, Z. Biomass derived 2D carbonsviaa hydrothermal carbonization method as efficient bifunctional ORR/HER electrocatalysts. J. Mater. Chem. A 2017, 5, 23481–23488. [Google Scholar] [CrossRef]
- Majidi, L.; Yasaei, P.; Warburton, R.E.; Fuladi, S.; Cavin, J.; Hu, X.; Hemmat, Z.; Cho, S.B.; Abbasi, P.; Vörös, M.; et al. New Class of Electrocatalysts Based on 2D Transition Metal Dichalcogenides in Ionic Liquid. Adv. Mater. 2018, 31, e1804453. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Bai, Z.; Lu, S. Recent Advances in Layered Ti3C2Tx MXene for Electrochemical Energy Storage. Small 2018, 14, e1703419. [Google Scholar] [CrossRef]
- Li, C.; Li, T.; Yu, G.; Chen, W. Theoretical Investigation of HER and OER Electrocatalysts Based on the 2D R-graphyne Completely Composed of Anti-Aromatic Carbon Rings. Molecules 2023, 28, 3888. [Google Scholar] [CrossRef]
- Zhang, Y.; Rui, K.; Ma, Z.; Sun, W.; Wang, Q.; Wu, P.; Zhang, Q.; Li, D.; Du, M.; Zhang, W.; et al. Cost-Effective Vertical Carbon Nanosheets/Iron-Based Composites as Efficient Electrocatalysts for Water Splitting Reaction. Chem. Mater. 2018, 30, 4762–4769. [Google Scholar] [CrossRef]
- Lei, Y.; Wei, L.; Zhai, S.; Wang, Y.; Karahan, H.E.; Chen, X.; Zhou, Z.; Wang, C.; Sui, X.; Chen, Y. Metal-free bifunctional carbon electrocatalysts derived from zeolitic imidazolate frameworks for efficient water splitting. Mater. Chem. Front. 2018, 2, 102–111. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, Q.; Zheng, H.; Chen, M.; Dai, L. Novel MOF-Derived Co@N-C Bifunctional Catalysts for Highly Efficient Zn–Air Batteries and Water Splitting. Adv. Mater. 2018, 30, 1705431. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.R.; Hong, Q.L.; Li, Q.H.; Du, Y.; Zhang, H.X.; Chen, S.; Zhou, T.; Zhang, J. Cobalt Boron Imidazolate Framework Derived Cobalt Nanoparticles Encapsulated in B/N Codoped Nanocarbon as Efficient Bifunctional Electrocatalysts for Overall Water Splitting. Adv. Funct. Mater. 2018, 28, 1801136. [Google Scholar] [CrossRef]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Metal–Organic Framework Derived Hybrid Co3O4-Carbon Porous Nanowire Arrays as Reversible Oxygen Evolution Electrodes. J. Am. Chem. Soc. 2014, 136, 13925–13931. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.L.; Zhao, M.Q.; Yu, D.; Kong, X.Y.; Huang, J.Q.; Zhang, Q.; Wei, F. Nitrogen-Doped Graphene/Carbon Nanotube Hybrids: In Situ Formation on Bifunctional Catalysts and Their Superior Electrocatalytic Activity for Oxygen Evolution/Reduction Reaction. Small 2014, 10, 2251–2259. [Google Scholar] [CrossRef]
- Wang, H.-F.; Tang, C.; Zhang, Q. Template growth of nitrogen-doped mesoporous graphene on metal oxides and its use as a metal-free bifunctional electrocatalyst for oxygen reduction and evolution reactions. Catal. Today 2018, 301, 25–31. [Google Scholar] [CrossRef]
- Lei, C.; Wang, Y.; Hou, Y.; Liu, P.; Yang, J.; Zhang, T.; Zhuang, X.; Chen, M.; Yang, B.; Lei, L.; et al. Efficient alkaline hydrogen evolution on atomically dispersed Ni–Nx Species anchored porous carbon with embedded Ni nanoparticles by accelerating water dissociation kinetics. Energy Environ. Sci. 2019, 12, 149–156. [Google Scholar] [CrossRef]
- Hou, Y.; Qiu, M.; Kim, M.G.; Liu, P.; Nam, G.; Zhang, T.; Zhuang, X.; Yang, B.; Cho, J.; Chen, M.; et al. Atomically dispersed nickel–nitrogen–sulfur species anchored on porous carbon nanosheets for efficient water oxidation. Nat. Commun. 2019, 10, 1392. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, J.; Xiao, H.; Hu, T.; Jia, J.; Wu, H. Facile in situ synthesis of a carbon quantum dot/graphene heterostructure as an efficient metal-free electrocatalyst for overall water splitting. Chem. Commun. 2019, 55, 1635–1638. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, X.; Yan, F.; Li, C.; Zhang, X.; Chen, Y. N-Doped graphene-supported Co@CoO core–shell nanoparticles as high-performance bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2016, 4, 12046–12053. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Huang, S.; Hou, Y.; Li, Y.; Zhao, Y.; Mu, S.; Zhang, J.; Zhao, Y. N,B-codoped defect-rich graphitic carbon nanocages as high performance multifunctional electrocatalysts. Nano Energy 2017, 42, 334–340. [Google Scholar] [CrossRef]
- Lyu, D.; Mollamahale, Y.B.; Huang, S.; Zhu, P.; Zhang, X.; Du, Y.; Wang, S.; Qing, M.; Tian, Z.Q.; Shen, P.K. Ultra-high surface area graphitic Fe-N-C nanospheres with single-atom iron sites as highly efficient non-precious metal bifunctional catalysts towards oxygen redox reactions. J. Catal. 2018, 368, 279–290. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, H.; Tan, X.; Guo, L.; Zhang, J.; Liu, S.; Guo, Y.; Zhang, J.; Wang, H.; Chu, W. Monoclinic ZIF-8 Nanosheet-Derived 2D Carbon Nanosheets as Sulfur Immobilizer for High-Performance Lithium Sulfur Batteries. ACS Appl. Mater. Interfaces 2017, 9, 25239–25249. [Google Scholar] [CrossRef]
- Low, J.; Cao, S.; Yu, J.; Wageh, S. Two-dimensional layered composite photocatalysts. Chem. Commun. 2014, 50, 10768–10777. [Google Scholar] [CrossRef]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Emerging Two-Dimensional Nanomaterials for Electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [CrossRef]
- Yu, H.; Ke, J.; Shao, Q. Two Dimensional Ir-Based Catalysts for Acidic OER. Small 2023, 19, e2304307. [Google Scholar] [CrossRef]
- Guo, Y.; Yuan, P.; Zhang, J.; Xia, H.; Cheng, F.; Zhou, M.; Li, J.; Qiao, Y.; Mu, S.; Xu, Q. Co2P–CoN Double Active Centers Confined in N-Doped Carbon Nanotube: Heterostructural Engineering for Trifunctional Catalysis toward HER, ORR, OER, and Zn–Air Batteries Driven Water Splitting. Adv. Funct. Mater. 2018, 28, 1805641. [Google Scholar] [CrossRef]
- Vijayakumar, E.; Ramakrishnan, S.; Sathiskumar, C.; Yoo, D.J.; Balamurugan, J.; Noh, H.S.; Kwon, D.; Kim, Y.H.; Lee, H. MOF-derived CoP-nitrogen-doped carbon@NiFeP nanoflakes as an efficient and durable electrocatalyst with multiple catalytically active sites for OER, HER, ORR and rechargeable zinc-air batteries. Chem. Eng. J. 2022, 428, 131115. [Google Scholar] [CrossRef]
- Vílchez-Cózar, Á.; Armakola, E.; Gjika, M.; Visa, A.; Bazaga-García, M.; Olivera-Pastor, P.; Choquesillo-Lazarte, D.; Marrero-López, D.; Cabeza, A.; Colodrero, R.M.P.; et al. Exploiting the Multifunctionality of M2+/Imidazole–Etidronates for Proton Conductivity (Zn2+) and Electrocatalysis (Co2+, Ni2+) toward the HER, OER, and ORR. ACS Appl. Mater. Interfaces 2022, 14, 11273–11287. [Google Scholar] [CrossRef]
- Liu, J.; Hodes, G.; Yan, J.; Liu, S. Metal-doped Mo2C (metal = Fe, Co, Ni, Cu) as catalysts on TiO2 for photocatalytic hydrogen evolution in neutral solution. Chin. J. Catal. 2021, 42, 205–216. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Luyen Doan, T.L.; Prabhakaran, S.; Tran, D.T.; Kim, D.H.; Lee, J.H.; Kim, N.H. Hierarchical Co and Nb dual-doped MoS2 nanosheets shelled micro-TiO2 hollow spheres as effective multifunctional electrocatalysts for HER, OER, and ORR. Nano Energy 2021, 82, 105750. [Google Scholar] [CrossRef]
- Talib, S.H.; Lu, Z.; Yu, X.; Ahmad, K.; Bashir, B.; Yang, Z.; Li, J. Theoretical Inspection of M1/PMA Single-Atom Electrocatalyst: Ultra-High Performance for Water Splitting (HER/OER) and Oxygen Reduction Reactions (OER). ACS Catal. 2021, 11, 8929–8941. [Google Scholar] [CrossRef]
- Chen, S.; Huang, H.; Jiang, P.; Yang, K.; Diao, J.; Gong, S.; Liu, S.; Huang, M.; Wang, H.; Chen, Q. Mn-Doped RuO2 Nanocrystals as Highly Active Electrocatalysts for Enhanced Oxygen Evolution in Acidic Media. ACS Catal. 2019, 10, 1152–1160. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Xiao, W.; Wang, X.; Fu, Y.; Xu, G.; Li, Z.; Wu, Z.; Wang, L.; Ru, B. Co-doped hollow structured iron phosphide as highly efficient electrocatalyst toward hydrogen generation in wide pH range. J. Mater. Chem. A 2022, 10, 15155–15160. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Wang, Y.; Ye, C.; Du, Z.; Yu, H.; Liu, J.; Chen, L.; Su, B. Efficient etching of oxygen-incorporated molybdenum disulfide nanosheet arrays for excellent electrocatalytic hydrogen evolution. Appl. Surf. Sci. 2019, 491, 245–255. [Google Scholar] [CrossRef]
- Gao, T.; Kumar, K.S.; Yan, Z.; Marinova, M.; Trentesaux, M.; Amin, M.A.; Szunerits, S.; Zhou, Y.; Martin-Diaconescu, V.; Paul, S.; et al. Covalent organic framework derived synthesis of Ru embedded in carbon nitride for hydrogen and oxygen evolution reactions. J. Mater. Chem. A 2023, 11, 19338–19348. [Google Scholar] [CrossRef]
- You, M.; Du, X.; Hou, X.; Wang, Z.; Zhou, Y.; Ji, H.; Zhang, L.; Zhang, Z.; Yi, S.; Chen, D. In-situ growth of ruthenium-based nanostructure on carbon cloth for superior electrocatalytic activity towards HER and OER. Appl. Catal. B Environ. 2022, 317, 121729. [Google Scholar] [CrossRef]
- Thangavel, P.; Ha, M.; Kumaraguru, S.; Meena, A.; Singh, A.N.; Harzandi, A.M.; Kim, K.S. Graphene-nanoplatelets-supported NiFe-MOF: High-efficiency and ultra-stable oxygen electrodes for sustained alkaline anion exchange membrane water electrolysis. Energy Environ. Sci. 2020, 13, 3447–3458. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Xia, Z.; Luo, M.; Zhang, Q.; Qin, Y.; Tao, L.; Yin, K.; Chao, Y.; Gu, L.; et al. Exclusive Strain Effect Boosts Overall Water Splitting in PdCu/Ir Core/Shell Nanocrystals. Angew. Chem. Int. Ed. 2021, 60, 8243–8250. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Q. Redox-Mediated Water Splitting for Decoupled H2 Production. ACS Mater. Lett. 2021, 3, 641–651. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Yu, A.; Zhang, L.; Zhang, J. A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ. Sci. 2011, 4, 3167–3192. [Google Scholar] [CrossRef]
- Feng, J.-X.; Tong, S.-Y.; Tong, Y.-X.; Li, G.-R. Pt-like Hydrogen Evolution Electrocatalysis on PANI/CoP Hybrid Nanowires by Weakening the Shackles of Hydrogen Ions on the Surfaces of Catalysts. J. Am. Chem. Soc. 2018, 140, 5118–5126. [Google Scholar] [CrossRef]
- Li, T.-T.; Dang, L.-L.; Zhao, C.-C.; Lv, Z.-Y.; Yang, X.-G.; Zhao, Y.; Zhang, S.-H. A self-sensitized Co (II)-MOF for efficient visible-light-driven hydrogen evolution without additional cocatalysts. J. Solid State Chem. 2021, 304, 122609. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhuo, S.; Liu, S.; Han, A.; Li, L.; Tian, Y. Flower-like CoO@Cu2S nanocomposite for enhanced oxygen evolution reaction. Appl. Surf. Sci. 2021, 555, 149441. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, D.; Wu, Q.; Fan, B.; Dan, J.; Han, A.; Ma, L.; Zhang, X.; Li, L. Amorphous CoP nanoparticle composites with nitrogen-doped hollow carbon nanospheres for synergetic anchoring and catalytic conversion of polysulfides in Li-S batteries. J. Colloid Interface Sci. 2021, 603, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lu, Y.; Wang, X.; Yu, H. Co-modification of amorphous-Ti(IV) hole cocatalyst and Ni(OH)2 electron cocatalyst for enhanced photocatalytic H2 -production performance of TiO2. Appl. Surf. Sci. 2017, 391, 259–266. [Google Scholar] [CrossRef]
- Wang, H.; Yang, P.; Sun, X.; Xiao, W.; Wang, X.; Tian, M.; Xu, G.; Li, Z.; Zhang, Y.; Liu, F.; et al. Co-Ru alloy nanoparticles decorated onto two-dimensional nitrogen doped carbon nanosheets towards hydrogen/oxygen evolution reaction and oxygen reduction reaction. J. Energy Chem. 2023, 87, 286–294. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Z.; Kong, X.; Xu, H.; Jin, W. Rational design of MOFs-derived Co-Ru species embedded N-doped carbon/carbon matrix for highly-efficient and multifunctional electrocatalysis. Appl. Surf. Sci. 2022, 606, 154818. [Google Scholar] [CrossRef]
- Ahn, M.; Cha, I.Y.; Cho, J.; Ham, H.C.; Sung, Y.-E.; Yoo, S.J. Rhodium–Tin Binary Nanoparticle—A Strategy to Develop an Alternative Electrocatalyst for Oxygen Reduction. ACS Catalysis 2017, 7, 5796–5801. [Google Scholar] [CrossRef]
- Tran, T.D.; Nguyen, M.T.T.; Le, H.V.; Nguyen, D.N.; Truong, Q.D.; Tran, P.D. Gold nanoparticles as an outstanding catalyst for the hydrogen evolution reaction. Chem. Commun. 2018, 54, 3363–3366. [Google Scholar] [CrossRef] [PubMed]
- Narwade, S.S.; Mali, S.M.; Sapner, V.S.; Sathe, B.R. Graphene Oxide Decorated with Rh Nanospheres for Electrocatalytic Water Splitting. ACS Appl. Nano Mater. 2020, 3, 12288–12296. [Google Scholar] [CrossRef]
- Li, T.; Kasian, O.; Cherevko, S.; Zhang, S.; Geiger, S.; Scheu, C.; Felfer, P.; Raabe, D.; Gault, B.; Mayrhofer, K.J.J. Atomic-scale insights into surface species of electrocatalysts in three dimensions. Nat. Catal. 2018, 1, 300–305. [Google Scholar] [CrossRef]
- Lettenmeier, P.; Wang, L.; Golla-Schindler, U.; Gazdzicki, P.; Cañas, N.A.; Handl, M.; Hiesgen, R.; Hosseiny, S.S.; Gago, A.S.; Friedrich, K.A. Nanosized IrOx–Ir Catalyst with Relevant Activity for Anodes of Proton Exchange Membrane Electrolysis Produced by a Cost-Effective Procedure. Angew. Chem. Int. Ed. 2015, 55, 742–746. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Ji, S.; Tang, Y.; Chen, W.; Li, A.; Zhao, J.; Xiong, Y.; Wu, Y.; Gong, Y.; et al. Iridium single-atom catalyst on nitrogen-doped carbon for formic acid oxidation synthesized using a general host–guest strategy. Nat. Chem. 2020, 12, 764–772. [Google Scholar] [CrossRef]
- Lebedev, D.; Ezhov, R.; Heras-Domingo, J.; Comas-Vives, A.; Kaeffer, N.; Willinger, M.; Solans-Monfort, X.; Huang, X.; Pushkar, Y.; Copéret, C. Atomically Dispersed Iridium on Indium Tin Oxide Efficiently Catalyzes Water Oxidation. ACS Cent. Sci. 2020, 6, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Zu, L.; Qian, X.; Zhao, S.; Liang, Q.; Chen, Y.E.; Liu, M.; Su, B.-J.; Wu, K.-H.; Qu, L.; Duan, L.; et al. Self-Assembly of Ir-Based Nanosheets with Ordered Interlayer Space for Enhanced Electrocatalytic Water Oxidation. J. Am. Chem. Soc. 2022, 144, 2208–2217. [Google Scholar] [CrossRef]
- Li, S.B.; Fei, B. Two-dimensional transition metal-based electrocatalyst and their application in water splitting. Mater. Sci. Technol. 2022, 9, 535–555. [Google Scholar] [CrossRef]
- Antolini, E. Iridium As Catalyst and Cocatalyst for Oxygen Evolution/Reduction in Acidic Polymer Electrolyte Membrane Electrolyzers and Fuel Cells. ACS Catal. 2014, 4, 1426–1440. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Qu, Z.W.; Zhu, H.; Kroes, G.J.; Nørskov, J.K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 2007, 607, 83–89. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, X.; Qin, J.; Liu, R. TMN4 complex embedded graphene as bifunctional electrocatalysts for high efficiency OER/ORR. J. Energy Chem. 2021, 55, 437–443. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, C.; Jian, H.; Cheng, X.; Hu, T.; Wang, D.; Shang, L.; Chen, G.; Schaaf, P.; Wang, X.; et al. Substitutionally Dispersed High-Oxidation CoOx Clusters in the Lattice of Rutile TiO2 Triggering Efficient Co—Ti Cooperative Catalytic Centers for Oxygen Evolution Reactions. Adv. Funct. Mater. 2020, 31, 2009610. [Google Scholar] [CrossRef]
- Zheng, X.; Cao, X.; Sun, Z.; Zeng, K.; Yan, J.; Strasser, P.; Chen, X.; Sun, S.; Yang, R. Indiscrete metal/metal-N-C synergic active sites for efficient and durable oxygen electrocatalysis toward advanced Zn-air batteries. Appl. Catal. B Environ. 2020, 272, 118967. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Jiang, G.; Li, G.; Zhu, J.; Xiao, M.; Zhu, Y.; Gao, R.; Yu, A.; Feng, M.; et al. Graphene Quantum Dots-Based Advanced Electrode Materials: Design, Synthesis and Their Applications in Electrochemical Energy Storage and Electrocatalysis. Adv. Energy Mater. 2020, 10, 2001275. [Google Scholar] [CrossRef]
- Wei, C.; Rao, R.R.; Peng, J.; Huang, B.; Stephens, I.E.L.; Risch, M.; Xu, Z.J.; Shao-Horn, Y. Recommended Practices and Benchmark Activity for Hydrogen and Oxygen Electrocatalysis in Water Splitting and Fuel Cells. Adv. Mater. 2019, 31, e1806296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lei, Y.; Chen, Z.; Wu, N.; Wang, Y.; Wang, B.; Wang, Y. Fe/Fe3C@C nanoparticles encapsulated in N-doped graphene–CNTs framework as an efficient bifunctional oxygen electrocatalyst for robust rechargeable Zn–air batteries. J. Mater. Chem. A 2018, 6, 516–526. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X.; Zhou, Z.-Y.; Yang, X.-D.; Zhang, X.-S.; Sun, S.-G. Highly active Fe, N co-doped graphene nanoribbon/carbon nanotube composite catalyst for oxygen reduction reaction. Electrochim. Acta 2016, 222, 1922–1930. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Li, Z.; Wang, L.; Niu, X.; Sun, P. Novel space-confinement synthesis of two-dimensional Fe, N-codoped graphene bifunctional oxygen electrocatalyst for rechargeable air-cathode. Chem. Eng. J. 2021, 411, 128492. [Google Scholar] [CrossRef]
- Li, C.; Yu, Z.; Liu, H.; Xiong, M. Synergetic contribution of Fe/Co and N/B dopants in mesoporous carbon nanosheets as remarkable electrocatalysts for zinc-air batteries. Chem. Eng. J. 2019, 371, 433–442. [Google Scholar] [CrossRef]
- Hui, L.; Xue, Y.; Huang, B.; Yu, H.; Zhang, C.; Zhang, D.; Jia, D.; Zhao, Y.; Li, Y.; Liu, H.; et al. Overall water splitting by graphdiyne-exfoliated and -sandwiched layered double-hydroxide nanosheet arrays. Nat. Commun. 2018, 9, 5309. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Li, Y.; Zuo, Z.; Liu, H.; Huang, C.; Li, Y. Synthesis and Properties of 2D Carbon—Graphdiyne. Acc. Chem. Res. 2017, 50, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, Y.; Zhang, J.; Liu, Z.; Zhao, Y. 2D graphdiyne materials: Challenges and opportunities in energy field. Sci. China Chem. 2018, 61, 765–786. [Google Scholar] [CrossRef]
- Yu, H.; Hui, L.; Xue, Y.; Liu, Y.; Fang, Y.; Xing, C.; Zhang, C.; Zhang, D.; Chen, X.; Du, Y.; et al. 2D graphdiyne loading ruthenium atoms for high efficiency water splitting. Nano Energy 2020, 72, 104667. [Google Scholar] [CrossRef]
- Yin, X.P.; Lu, D.; Wang, J.W.; Lu, X.L. 2D/2D Heterojunction of Ni−Co−P/Graphdiyne for Optimized Electrocatalytic Overall Water Splitting. ChemCatChem 2019, 11, 5407–5411. [Google Scholar] [CrossRef]
- Yin, W.-J.; Xie, Y.-E.; Liu, L.-M.; Wang, R.-Z.; Wei, X.-L.; Lau, L.; Zhong, J.-X.; Chen, Y.-P. R-graphyne: A new two-dimensional carbon allotrope with versatile Dirac-like point in nanoribbons. J. Mater. Chem. A 2013, 1, 5341–5346. [Google Scholar] [CrossRef]
- Wang, J.; Xu, F.; Jin, H.; Chen, Y.; Wang, Y. Non-Noble Metal-based Carbon Composites in Hydrogen Evolution Reaction: Fundamentals to Applications. Adv. Mater. 2017, 29, 1605838. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Carbon-Based Metal-Free Catalysts for Electrocatalysis beyond the ORR. Angew. Chem. Int. Ed. 2016, 55, 11736–11758. [Google Scholar] [CrossRef]
- Tian, G.L.; Zhang, Q.; Zhang, B.; Jin, Y.G.; Huang, J.Q.; Su, D.S.; Wei, F. Toward Full Exposure of “Active Sites”: Nanocarbon Electrocatalyst with Surface Enriched Nitrogen for Superior Oxygen Reduction and Evolution Reactivity. Adv. Funct. Mater. 2014, 24, 5956–5961. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, G.; Zhang, F. Multiple roles of graphene in heterogeneous catalysis. Chem. Soc. Rev. 2015, 44, 3023–3035. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, J.; Xiao, Y.; Wang, J.; Sun, G.; Zhao, Y.; Qu, L. Regulation of 2D Graphene Materials for Electrocatalysis. Chem. Asian J. 2020, 15, 2271–2281. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, J.; Yang, M.; Fang, Z.; Jian, J.; Yu, D.; Chen, X.; Dai, L. Ultrathin Black Phosphorus-on-Nitrogen Doped Graphene for Efficient Overall Water Splitting: Dual Modulation Roles of Directional Interfacial Charge Transfer. J. Am. Chem. Soc. 2019, 141, 4972–4979. [Google Scholar] [CrossRef]
- Choi, H.; Surendran, S.; Sim, Y.; Je, M.; Janani, G.; Choi, H.; Kim, J.K.; Sim, U. Enhanced electrocatalytic full water-splitting reaction by interfacial electric field in 2D/2D heterojunction. Chem. Eng. J. 2022, 450, 137789. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, S.; Yang, P.; Huang, C.; Wang, X. Boron Carbon Nitride Semiconductors Decorated with CdS Nanoparticles for Photocatalytic Reduction of CO2. ACS Catal. 2018, 8, 4928–4936. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, M.; Luo, Z.; Zhang, J.; Wang, S.; Wang, X. Molten salt assisted assembly growth of atomically thin boron carbon nitride nanosheets for photocatalytic H2 evolution. Chem. Commun. 2020, 56, 2558–2561. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, Z.; Yang, P.; Wang, S.; Huang, C.; Wang, X. Hydrogen reduction treatment of boron carbon nitrides for photocatalytic selective oxidation of alcohols. Appl. Catal. B Environ. 2020, 276, 118916. [Google Scholar] [CrossRef]
- Chen, M.; Guan, R.; Yang, S. Hybrids of Fullerenes and 2D Nanomaterials. Adv. Sci. 2018, 6, 1800941. [Google Scholar] [CrossRef]
- Ahsan, M.A.; He, T.; Eid, K.; Abdullah, A.M.; Curry, M.L.; Du, A.; Puente Santiago, A.R.; Echegoyen, L.; Noveron, J.C. Tuning the Intermolecular Electron Transfer of Low-Dimensional and Metal-Free BCN/C60 Electrocatalysts via Interfacial Defects for Efficient Hydrogen and Oxygen Electrochemistry. J. Am. Chem. Soc. 2021, 143, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gong, F.; Zhou, Q.; Feng, X.; Fan, J.; Xiang, Q. Crystalline isotype heptazine-/triazine-based carbon nitride heterojunctions for an improved hydrogen evolution. Appl. Catal. B Environ. 2020, 268, 118381. [Google Scholar] [CrossRef]
- Talapaneni, S.N.; Singh, G.; Kim, I.Y.; AlBahily, K.; Al-Muhtaseb, A.a.H.; Karakoti, A.S.; Tavakkoli, E.; Vinu, A. Nanostructured Carbon Nitrides for CO2 Capture and Conversion. Adv. Mater. 2019, 32, e1904635. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Huang, G.-F.; Li, D.-F.; Zhang, M.; Pan, A.-L.; Huang, W.-Q. Facile synthesis and superior photocatalytic and electrocatalytic performances of porous B-doped g-C3N4 nanosheets. J. Mater. Sci. Technol. 2018, 34, 2515–2520. [Google Scholar] [CrossRef]
- Zhang, H.-W.; Lu, Y.-X.; Li, B.; Huang, G.-F.; Zeng, F.; Li, Y.-Y.; Pan, A.; Chai, Y.-F.; Huang, W.-Q. Acid-induced topological morphology modulation of graphitic carbon nitride homojunctions as advanced metal-free catalysts for OER and pollutant degradation. J. Mater. Sci. Technol. 2021, 86, 210–218. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Guo, X.; Chen, Z.; Zhang, W.; Wang, Y.; Tang, X.; Zhang, Y.; Zhao, Y. Sulfur-doped g-C3N4/rGO porous nanosheets for highly efficient photocatalytic degradation of refractory contaminants. J. Mater. Sci. Technol. 2020, 41, 117–126. [Google Scholar] [CrossRef]
- Ma, T.; Bai, J.; Wang, Q.; Li, C. The novel synthesis of a continuous tube with laminated g-C3N4 nanosheets for enhancing photocatalytic activity and oxygen evolution reaction performance. Dalton Trans. 2018, 47, 10240–10248. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, D.; Lin, L.; Liu, Y.; Yuan, M.; Nan, C.; Li, H.; Sun, G.; Yang, X. Ultrathin hexagonal boron nitride as a van der Waals’ force initiator activated graphene for engineering efficient non-metal electrocatalysts of Li-CO2 battery. Nano Res. 2021, 15, 1171–1177. [Google Scholar] [CrossRef]
- Al–Naggar, A.H.; Shinde, N.M.; Kim, J.S.; Mane, R.S. Water splitting performance of metal and non-metal-doped transition metal oxide electrocatalysts. Coord. Chem. Rev. 2023, 474, 214864. [Google Scholar] [CrossRef]
- Ao, X.; Zhang, W.; Zhao, B.; Ding, Y.; Nam, G.; Soule, L.; Abdelhafiz, A.; Wang, C.; Liu, M. Atomically dispersed Fe–N–C decorated with Pt-alloy core–shell nanoparticles for improved activity and durability towards oxygen reduction. Energy Environ. Sci. 2020, 13, 3032–3040. [Google Scholar] [CrossRef]
- Li, Z.; Wu, X.; Jiang, X.; Shen, B.; Teng, Z.; Sun, D.; Fu, G.; Tang, Y. Surface carbon layer controllable Ni3Fe particles confined in hierarchical N-doped carbon framework boosting oxygen evolution reaction. Adv. Powder Mater. 2022, 1, 100020. [Google Scholar] [CrossRef]
- Tang, L.; Meng, X.; Deng, D.; Bao, X. Confinement Catalysis with 2D Materials for Energy Conversion. Adv. Mater. 2019, 31, e1901996. [Google Scholar] [CrossRef] [PubMed]
- Tareen, A.K.; Khan, K.; Aslam, M.; Liu, X.; Zhang, H. Confinement in two-dimensional materials: Major advances and challenges in the emerging renewable energy conversion and other applications. Prog. Solid State Chem. 2021, 61, 100294. [Google Scholar] [CrossRef]
- Du, J.; Li, F.; Sun, L. Metal–organic frameworks and their derivatives as electrocatalysts for the oxygen evolution reaction. Chem. Soc. Rev. 2021, 50, 2663–2695. [Google Scholar] [CrossRef] [PubMed]
- Li, C.F.; Xie, L.J.; Zhao, J.W.; Gu, L.F.; Tang, H.B.; Zheng, L.; Li, G.R. Interfacial Fe−O−Ni−O−Fe Bonding Regulates the Active Ni Sites of Ni-MOFs via Iron Doping and Decorating with FeOOH for Super-Efficient Oxygen Evolution. Angew. Chem. Int. Ed. 2022, 61, e202116934. [Google Scholar] [CrossRef]
- Li, S.; Gao, Y.; Li, N.; Ge, L.; Bu, X.; Feng, P. Transition metal-based bimetallic MOFs and MOF-derived catalysts for electrochemical oxygen evolution reaction. Energy Environ. Sci. 2021, 14, 1897–1927. [Google Scholar] [CrossRef]
- Lyu, S.; Guo, C.; Wang, J.; Li, Z.; Yang, B.; Lei, L.; Wang, L.; Xiao, J.; Zhang, T.; Hou, Y. Exceptional catalytic activity of oxygen evolution reaction via two-dimensional graphene multilayer confined metal-organic frameworks. Nat. Commun. 2022, 13, 6171. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Zhang, X.; Sang, S.-Y.; Zhang, L.; Feng, J.-J.; Wang, A.-J. Bio-derived FeNi alloy confined in N-doped carbon nanosheets as efficient air electrodes for Zn–air battery. J. Colloid Interface Sci. 2022, 628, 499–507. [Google Scholar] [CrossRef]
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.; Wang, H.-L.; Dai, L. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition. Nano Energy 2016, 29, 83–110. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, J.; Lin, H.-F.; Tang, P.; Morante, J.R.; Arbiol, J.; Wan, K.; Mao, B.-W.; Liu, L.-M.; Fransaer, J. Tailor-made metal-nitrogen-carbon bifunctional electrocatalysts for rechargeable Zn-air batteries via controllable MOF units. Energy Storage Mater. 2019, 17, 46–61. [Google Scholar] [CrossRef]
- Xi, W.; Shen, M.; Yin, X.; Gao, B.; He, L.; Chen, Y.; Lin, B. Molten-salt confined synthesis of nitrogen-doped carbon nanosheets supported Co3O4 nanoparticles as a superior oxygen electrocatalyst for rechargeable Zn-air battery. J. Power Sources 2023, 560. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.D.; Cheng, Y.H.; Huang, Y.; Chen, C. Covalent organic polymer derived N-doped carbon confined FeNi alloys as bifunctional oxygen electrocatalyst for rechargeable zinc-air battery. Int. J. Hydrogen Energy. 2022, 47, 16025–16035. [Google Scholar] [CrossRef]
- Younis, M.A.; Lyu, S.; Zhao, Q.; Lei, C.; Zhang, P.; Yang, B.; Li, Z.; Lei, L.; Hou, Y.; Feng, X. Noble metal-free two dimensional carbon-based electrocatalysts for water splitting. BMC Mater. 2019, 1, 6. [Google Scholar] [CrossRef]
- Kim, H.T.; Shin, H.; Jeon, I.Y.; Yousaf, M.; Baik, J.; Cheong, H.W.; Park, N.; Baek, J.B.; Kwon, T.H. Carbon–Heteroatom Bond Formation by an Ultrasonic Chemical Reaction for Energy Storage Systems. Adv. Mater. 2017, 29, 1702747. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, X.; Li, Z.; Wang, J.H.; Zhu, L.; Yin, C.; Wang, Y.; Li, X.B.; Liu, Z.; Zhang, J.; et al. Superhydrophilic Graphdiyne Accelerates Interfacial Mass/Electron Transportation to Boost Electrocatalytic and Photoelectrocatalytic Water Oxidation Activity. Adv. Funct. Mater. 2019, 29, 1808079. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, H.; Zhang, X.; Pan, J.; Dong, Y.; Wang, H.; Gao, Y. Magnetic N-containing carbon spheres derived from sustainable chitin for the selective oxidation of C–H bonds. RSC Adv. 2017, 7, 51831–51837. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wu, M.; Wei, Z.; Cui, C.; Mao, M.; Zhang, J.; Han, X.; Liu, Q.; Ma, J. Nitrogen, Fluorine, and Boron Ternary Doped Carbon Fibers as Cathode Electrocatalysts for Zinc–Air Batteries. Small 2018, 14, e1800737. [Google Scholar] [CrossRef]
- Yang, C.C.; Zai, S.F.; Zhou, Y.T.; Du, L.; Jiang, Q. Fe3C-Co Nanoparticles Encapsulated in a Hierarchical Structure of N-Doped Carbon as a Multifunctional Electrocatalyst for ORR, OER, and HER. Adv. Funct. Mater. 2019, 29, 1901949. [Google Scholar] [CrossRef]
- Ke, J.; Adnan Younis, M.; Kong, Y.; Zhou, H.; Liu, J.; Lei, L.; Hou, Y. Nanostructured Ternary Metal Tungstate-Based Photocatalysts for Environmental Purification and Solar Water Splitting: A Review. Nano-Micro Lett. 2018, 10, 69. [Google Scholar] [CrossRef]
- Lu, C.; Yang, J.; Wei, S.; Bi, S.; Xia, Y.; Chen, M.; Hou, Y.; Qiu, M.; Yuan, C.; Su, Y.; et al. Atomic Ni Anchored Covalent Triazine Framework as High Efficient Electrocatalyst for Carbon Dioxide Conversion. Adv. Funct. Mater. 2019, 29, 1806884. [Google Scholar] [CrossRef]
- Peng, X.; Pi, C.; Zhang, X.; Li, S.; Huo, K.; Chu, P.K. Recent progress of transition metal nitrides for efficient electrocatalytic water splitting. Sustain. Energy Fuels 2019, 3, 366–381. [Google Scholar] [CrossRef]
- Liu, T.; Li, P.; Yao, N.; Kong, T.; Cheng, G.; Chen, S.; Luo, W. Self-Sacrificial Template-Directed Vapor-Phase Growth of MOF Assemblies and Surface Vulcanization for Efficient Water Splitting. Adv. Mater. 2019, 31, 1806672. [Google Scholar] [CrossRef] [PubMed]




| Catalyst | Carbon Precursor | Precious Metal | Electrolyte | Overpotential (mV@10 mA cm−2) | Tafel Slope (mV dec−1) | Surface Area (m2 g−1) | Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ru@g−CNx | graphitic carbon nitride | Ru | 1 M KOH | 280 | 49.5 | 23 | 45 h | [42] |
| Ru−H2O/CC−350 | CC substrate | Ru | 1 M KOH | 270 | 63 | — | 100 h | [43] |
| Co−Ru@RuOx/NCN | NCN | Ru | 1 M KOH | 270 | 48 | 603.45 | 12 h | [53] |
| Ru@RuOx/NCN | NCN | Ru | 1 M KOH | 310 | — | — | — | [53] |
| CoRuOx@C | N−doped carbon matrix | Ru | 1 M KOH | 240 | 61.8 | — | — | [54] |
| Rh−GO | GO | Rh | 0.5 M KOH | 230 | 27 | 8.909 | — | [57] |
| Ir−IrOx/C−20 | C−20 | Ir−IrOx | 0.5 M H2SO4 | 198 | 106.3 | 146 | 18 | [62] |
| Ir−IrOx/C−10 | C−10 | Ir−IrOx | 0.5 M H2SO4 | — | 257.2 | 182 | — | [62] |
| Ir−IrOx/C−30 | C−30 | Ir−IrOx | 0.5 M H2SO4 | — | 115.1 | 114 | — | [62] |
| Catalyst | Carbon Precursor | Doping Material | Electrolyte | Overpotential (mV@10 mA cm−2) | Tafel Slope (mV dec−1) | Surface Area (m2 g−1) | Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ni@R−graphyne | R-graphyne | Ni | — | 310 | — | — | — | [15] |
| Fe−NG | graphene | Fe, N | 0.1 M KOH | 390 | 70.1 | 714.5097 | 80h | [73] |
| NG | graphene | N | 0.1 M KOH | 403 | 71.3 | 563.7250 | — | [73] |
| FeCo/NB−Cs | mesoporous carbon nanosheets | Fe−Co and N−B | 0.1 M KOH | 653 | — | 1584 | 6700 min | [74] |
| Fe/N−Cs | mesoporous carbon nanosheets | Fe | 0.1 M KOH | 745 | — | 1235 | — | [74] |
| Fe/NB−Cs | mesoporous carbon nanosheets | Fe | 0.1 M KOH | 729 | — | 1654 | — | [74] |
| Ni−Co−P/GDY | GDY | Ni−Co−P nanosheets | 1 M KOH | 290 | 72.7 | — | 45 h | [79] |
| EBP@NG(1:4) | NG | EBP | 1 M KOH | 350 | 82 | — | 16 h | [86] |
| EBP@NG(1:8) | NG | EBP | 1 M KOH | 310 | 89 | — | — | [86] |
| g−C3N4/rGO | rGO | g−C3N4 | 1 M KOH | 272 | 97 | 142.49 | 24 h | [87] |
| 10% F/BCN | CN | F, B | 0.5 M NaOH | 390 | 79 | — | 12 h | [92] |
| CNS−0.5N | CN | S | 1 M KOH | 301 | 57.71 | — | — | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Niu, S.; Xi, B.; Du, Z.; Yu, T.; Wan, T.; Lei, C.; Lyu, S. Recent Developments in Two-Dimensional Carbon-Based Nanomaterials for Electrochemical Water Oxidation: A Mini Review. Catalysts 2024, 14, 221. https://doi.org/10.3390/catal14040221
Zhao Y, Niu S, Xi B, Du Z, Yu T, Wan T, Lei C, Lyu S. Recent Developments in Two-Dimensional Carbon-Based Nanomaterials for Electrochemical Water Oxidation: A Mini Review. Catalysts. 2024; 14(4):221. https://doi.org/10.3390/catal14040221
Chicago/Turabian StyleZhao, Yuxin, Siyuan Niu, Baichuan Xi, Zurong Du, Ting Yu, Tongtao Wan, Chaojun Lei, and Siliu Lyu. 2024. "Recent Developments in Two-Dimensional Carbon-Based Nanomaterials for Electrochemical Water Oxidation: A Mini Review" Catalysts 14, no. 4: 221. https://doi.org/10.3390/catal14040221
APA StyleZhao, Y., Niu, S., Xi, B., Du, Z., Yu, T., Wan, T., Lei, C., & Lyu, S. (2024). Recent Developments in Two-Dimensional Carbon-Based Nanomaterials for Electrochemical Water Oxidation: A Mini Review. Catalysts, 14(4), 221. https://doi.org/10.3390/catal14040221