Stably Improving the Catalytic Activity of Oxygen Evolution Reactions via Two-Dimensional Graphene Oxide-Incorporated NiFe-Layered Double Hydroxides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Material Synthesis and Characterization
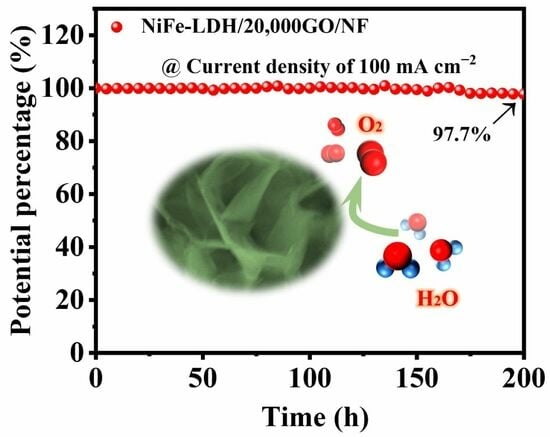
2.2. Electrochemical Activity towards OER
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of NiFe-LDH/GO/NF
3.3. Material Characterization
3.4. Oxygen Evolution Reaction Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Munonde, T.S.; Zheng, H.T.; Nomngongo, P.N. Ultrasonic exfoliation of NiFe LDH/CB nanosheets for enhanced oxygen evolution catalysis. Ultrason. Sonochem. 2019, 59, 104716. [Google Scholar] [CrossRef]
- Bai, L.; Song, A.; Lei, X.; Zhang, T.; Song, S.; Tian, H.; Liu, H.; Qin, X.; Wang, G.; Shao, G. Hierarchical construction of hollow NiCo2S4 nanotube@NiCo2S4 nanosheet arrays on Ni foam as an efficient and durable electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 38524. [Google Scholar] [CrossRef]
- Song, A.; Song, S.; Duanmu, M.; Tian, H.; Liu, H.; Qin, X.; Shao, G.; Wang, G. Recent Progress of Non-Noble Metallic Heterostructures for the Electrocatalytic Hydrogen Evolution. Small Sci. 2023, 3, 2300036. [Google Scholar] [CrossRef]
- Song, S.; Song, A.; Bai, L.; Duanmu, M.; Wang, L.; Dong, H.; Qin, X.; Shao, G. Hierarchical Design of Homologous NiCoP/NF from Layered Double Hydroxides as a Long-Term Stable Electrocatalyst for Hydrogen Evolution. Catalysts 2023, 13, 1232. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Shao, Z.; Jung, W. Advanced electrocatalysts with unusual active sites for electrochemical water splitting. InfoMat 2023, 6, e12494. [Google Scholar] [CrossRef]
- Pan, S.; Ma, Z.; Yang, W.; Dongyang, B.; Yang, H.; Lai, S.; Dong, F.; Yang, X.; Lin, Z. Magnesium incorporation activates perovskite cobaltites toward efficient and stable electrocatalytic oxygen evolution. Mater. Rep. Energy 2023, 3, 100212. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Q.N.; Yang, Y.B.; Guo, P.F.; Feng, W.X.; Jia, Y.; Wang, K.; Wang, W.T.; He, Z.H.; Liu, Z.T. Enhancing Water Oxidation of Ru Single Atoms via Oxygen- Coordination Bonding with NiFe Layered Double Hydroxide. ACS Catal. 2023, 13, 2771–2779. [Google Scholar] [CrossRef]
- Liu, S.X.; Zhang, H.W.; Hu, E.L.; Zhu, T.Y.; Zhou, C.Y.; Huang, Y.C.; Ling, M.; Gao, X.H.; Lin, Z. Boosting oxygen evolution activity of NiFe-LDH using oxygen vacancies and morphological engineering. J. Mater. Chem. A 2021, 9, 23697. [Google Scholar] [CrossRef]
- Hu, J.; Liang, Y.Q.; Wu, S.L.; Li, Z.Y.; Shi, C.S.; Luo, S.Y.; Sun, H.J.; Zhu, S.L.; Cui, Z.D. Hierarchical nickle-iron layered double hydroxide composite electrocatalyst for efficient oxygen evolution reaction. Mater. Today Nano 2022, 17, 100150. [Google Scholar] [CrossRef]
- Shen, K.; Tang, Y.; Zhou, Q.; Zhang, Y.; Ge, W.; Shai, X.; Deng, S.; Yang, P.; Deng, S.; Wang, J. Metal-organic framework-derived S-NiFe PBA coupled with NiFe layered double hydroxides as Mott-Schottky electrocatalysts for efficient alkaline oxygen evolution reaction. Chem. Eng. J. 2023, 471, 144827. [Google Scholar] [CrossRef]
- Zhao, J.; Liao, N.; Luo, J. Transforming NiFe layered double hydroxide into NiFePx for efficient alkaline water splitting. J. Mater. Chem. A 2023, 11, 9682. [Google Scholar] [CrossRef]
- Xu, H.; Xin, G.; Hu, W.; Zhang, Z.; Si, C.; Chen, J.; Lu, L.; Peng, Y.; Li, X. Single-atoms Ru/NiFe layered double hydroxide electrocatalyst: Efficient for oxidation of selective oxidation of 5-hydroxymethylfurfural and oxygen evolution reaction. Appl. Catal. B Environ. 2023, 339, 123157. [Google Scholar] [CrossRef]
- Yin, X.; Hua, Y.N.; Gao, Z. In situ construction of S-doped NiFe-layered double hydroxide nanoarrays on porous reduced graphene oxide as efficient oxygen evolution electrocatalysts for electrolytic cells. J. Energy Storage 2023, 73, 109102. [Google Scholar] [CrossRef]
- Liao, Y.Y.; He, R.C.; Pan, W.H.; Li, Y.; Wang, Y.Y.; Li, J.; Li, Y.X. Lattice distortion induced Ce-doped NiFe-LDH for efficient oxygen evolution. Chem. Eng. J. 2023, 464, 142669. [Google Scholar] [CrossRef]
- Liu, S.L.; Wan, R.D.; Lin, Z.S.; Liu, Z.; Liu, Y.G.; Tian, Y.; Qin, D.D.; Tang, Z.H. Probing the Co role in promoting the OER and Zn-air battery performance of NiFe-LDH: A combined experimental and theoretical study. J. Mater. Chem. A 2022, 10, 5244. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Gao, Y.P.; Ma, L.X.; Xue, Y.Z.; Liu, Z.H.; Cui, H.L.; Zhang, N.; Jiang, R.B. Atomically Dispersed Fe-N4 Sites and NiFe-LDH Sub-Nanoclusters as an Excellent Air Cathode for Rechargeable Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2023, 15, 16732. [Google Scholar] [CrossRef]
- Munonde, T.S.; Zheng, H.T. The impact of ultrasonic parameters on the exfoliation of NiFe LDH nanosheets as electrocatalysts for the oxygen evolution reaction in alkaline media. Ultrason. Sonochem. 2021, 76, 105664. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, H.; Zhang, Y.; Zhang, H.; Zhao, J.; Song, R. Hollow Mo-doped NiSx nanoarrays decorated with NiFe layered double-hydroxides for efficient and stable overall water splitting. J. Mater. Chem. A 2022, 10, 18989. [Google Scholar] [CrossRef]
- Li, W.M.; Chen, S.H.; Zhong, M.X.; Wang, C.; Lu, X.F. Synergistic coupling of NiFe layered double hydroxides with Co-C nanofibers for high-efficiency oxygen evolution reaction. Chem. Eng. J. 2021, 415, 128879. [Google Scholar] [CrossRef]
- Dresp, S.; Dionigi, F.; Klingenhof, M.; Merzdorf, T.; Schmies, H.; Drnec, J.; Poulain, A.; Strasser, P. Molecular Understanding of the Impact of Saline Contaminants and Alkaline pH on NiFe Layered Double Hydroxide Oxygen Evolution Catalysts. ACS Catal. 2021, 11, 6800. [Google Scholar] [CrossRef]
- Rong, M.K.; Mo, Y.; Cao, Z.F.; Ma, X.; Wang, S.; Zhong, H. MoSe2 regulates Ce-doped NiFe layered double hydroxide for efficient oxygen evolution reaction: The increase of active sites. Int. J. Hydrogen Energy 2022, 47, 18688. [Google Scholar] [CrossRef]
- Xie, Q.X.; Ren, D.; Bai, L.C.; Ge, R.L.; Zhou, W.H.; Bai, L.; Xie, W.; Wang, J.H.; Graetzel, M.; Luo, J.S. Investigation of nickel iron layered double hydroxide for water oxidation in different pH electrolytes. Chin. J. Catal. 2023, 44, 127. [Google Scholar] [CrossRef]
- Shi, K.; Sun, Z.; Yuan, M.; Zhao, Y.; Sun, G. “Polyoxometalate electron sponge” induces the accurate regulation of electron states at Ni sites to enhance oxidation of water. J. Colloid Interface Sci. 2023, 657, 37. [Google Scholar] [CrossRef] [PubMed]
- Arshad, F.; Munir, A.; Tahir, A.; Hussain, S.Z.; Jilani, A.; Hussain, A.; Ullah, N.; Sher, F.; Hussain, I. Microwave-assisted growth of spherical core-shell NiFe LDH@CuxO nanostructures for electrocatalytic water oxidation reaction. Int. J. Hydrogen Energy 2023, 48, 4719. [Google Scholar] [CrossRef]
- Wen, Q.L.; Wang, S.Z.; Wang, R.W.; Huang, D.J.; Fang, J.K.; Liu, Y.W.; Zhai, T.Y. Nanopore-rich NiFe LDH targets the formation of the high-valent nickel for enhanced oxygen evolution reaction. Nano Res. 2023, 16, 2286. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Yuan, X.; Dong, W.; Huang, F. Rational composition and structural design of in situ grown nickel-based electrocatalysts for efficient water electrolysis. J. Mater. Chem. A 2016, 4, 167. [Google Scholar] [CrossRef]
- Chen, Z.W.; Ju, M.; Sun, M.Z.; Jin, L.; Cai, R.M.; Wang, Z.; Dong, L.; Peng, L.M.; Long, X.; Huang, B.L.; et al. TM LDH Meets Birnessite: A 2D-2D Hybrid Catalyst with Long-Term Stability for Water Oxidation at Industrial Operating Conditions. Angew. Chem. -Int. Ed. 2021, 60, 9699. [Google Scholar] [CrossRef]
- Huang, J.W.; Li, K.; Wang, L.; She, H.D.; Wang, Q.Z. In situ conversion builds MIL-101@NiFe-LDH heterojunction structures to enhance the oxygen evolution reaction. Chin. Chem. Lett. 2022, 33, 3787. [Google Scholar] [CrossRef]
- Dong, Y.; Komarneni, S.; Wang, N.; Hu, W.C.; Huang, W.Y. An in situ anion exchange induced high-performance oxygen evolution reaction catalyst for the pH-near-neutral potassium borate electrolyte. J. Mater. Chem. A 2019, 7, 6995. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J. A review on NiFe-based electrocatalysts for efficient alkaline oxygen evolution reaction. J. Power Sources 2020, 448, 227375. [Google Scholar] [CrossRef]
- Li, S.Z.; Liu, J.Y.; Duan, S.; Wang, T.Y.; Li, Q. Tuning the oxygen evolution electrocatalysis on NiFe-layered double hydroxides via sulfur doping. Chin. J. Catal. 2020, 41, 847. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Zhu, X.H.; Wu, Z.X.; Zhao, D.S.; Wang, F.; Sun, D.M.; Tang, Y.W.; Li, H.; Fu, G.T. Improving the Oxygen Evolution Activity of Layered Double-Hydroxide via Erbium-Induced Electronic Engineering. Small 2023, 19, e2206531. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Guo, T.; Lei, X.; Guo, S.; Liu, Q.; Lu, J.; Zhang, T. Enhancing Electrocatalytic Water Oxidation of NiFe-LDH Nanosheets via Bismuth-Induced Electronic Structure Engineering. ACS Appl. Mater. Interfaces 2023, 15, 58784. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Q.; Deng, H.Y.; Feng, H.R.; Luo, G.Q.; Tu, R.; Zhang, L.M. Triethanolamine-assisted synthesis of NiFe layered double hydroxide ultrathin nanosheets for efficient oxygen evolution reaction. J. Colloid Interface Sci. 2023, 629, 610. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.S.; Zhang, B.; Ruan, Y.J.; Lv, L.; Ji, X.; Xu, K.; Miao, L.; Jiang, J.J. Hierarchical NiCo2S4@NiFe LDH Heterostructures Supported on Nickel Foam for Enhanced Overall-Water-Splitting Activity. ACS Appl. Mater. Interfaces 2017, 9, 15364. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, H.; Jiang, X.C.; Jiang, M.Q.; Zhan, X.; Fu, G.T.; Lee, J.M.; Tang, Y.W. Gd-induced electronic structure engineering of a NiFe-layered double hydroxide for efficient oxygen evolution. J. Mater. Chem. A 2021, 9, 2999. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, P.; Xie, R.S.; Chen, L.; Li, M.T.; Li, J.P.; Ji, B.Q.; Hu, Z.Y.; Li, J.J.; Song, L.X.; et al. Controlled Self-Assembled NiFe Layered Double Hydroxides/Reduced Graphene Oxide Nanohybrids Based on the Solid-Phase Exfoliation Strategy as an Excellent Electrocatalyst for the Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 13545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, X.; Luo, H.; Feng, X.; Chen, H. Design of wood-based self-supporting metal catalyst based on NiCo2O4 bridge for efficient oxygen evolution. Chem. Eng. J. 2023, 477, 147289. [Google Scholar] [CrossRef]
- Shen, B.; Feng, Y.; Wang, Y.; Sun, P.; Yang, L.; Jiang, Q.; He, H.; Huang, H. Holey MXene nanosheets intimately coupled with ultrathin Ni-Fe layered double hydroxides for boosted hydrogen and oxygen evolution reactions. Carbon 2023, 212, 118141. [Google Scholar] [CrossRef]
- Feng, X.; Jiao, Q.; Chen, W.; Dang, Y.; Dai, Z.; Suib, S.L.; Zhang, J.; Zhao, Y.; Li, H.; Feng, C. Cactus-like NiCo2S4@NiFe LDH hollow spheres as an effective oxygen bifunctional electrocatalyst in alkaline solution. Appl. Catal. B Environ. 2021, 286, 119869. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Xu, W.W.; Zhu, W.; Yang, Q.; Lei, X.D.; Liu, J.F.; Li, Y.P.; Sun, X.M.; Duan, X. Three-dimensional NiFe layered double hydroxide film for high-efficiency oxygen evolution reaction. Chem. Commun. 2014, 50, 6479. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pan, Y.; Ge, L.; Chen, Y.; Mao, X.; Guan, D.; Li, M.; Zhong, Y.; Hu, Z.; Peterson, V.K.; et al. High-Performance Perovskite Composite Electrocatalysts Enabled by Controllable Interface Engineering. Small 2021, 17, 2101573. [Google Scholar] [CrossRef]
- Du, Y.; Liu, D.; Li, T.; Yan, Y.; Liang, Y.; Yan, S.; Zou, Z. A phase transformation-free redox couple mediated electrocatalytic oxygen evolution reaction. Appl. Catal. B Environ. 2022, 306, 121146. [Google Scholar] [CrossRef]
- Cao, S.; Huang, H.; Shi, K.; Wei, L.; You, N.; Fan, X.; Yang, Z.; Zhang, W. Engineering superhydrophilic/superaerophobic hierarchical structures of Co-CH@NiFe-LDH/NF to boost the oxygen evolution reaction. Chem. Eng. J. 2021, 422, 130123. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, Y.; Xing, Y.; Li, D.; Jiang, D.; Chen, M.; Shi, W.; Yuan, S. Synergistic coupling of CoFe-LDH arrays with NiFe-LDH nanosheet for highly efficient overall water splitting in alkaline media. Appl. Catal. B Environ. 2019, 253, 131. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, Y.; Yu, Z.; Guo, S.; Yuan, M.; Yao, H.; Liang, Z.; Lu, Y.R.; Chan, T.S.; Li, C.; et al. Self-supported NiFe-LDH@CoSx nanosheet arrays grown on nickel foam as efficient bifunctional electrocatalysts for overall water splitting. Chem. Eng. J. 2021, 419, 129512. [Google Scholar] [CrossRef]
- Hu, L. Modulating interfacial electronic structure of CoNi LDH nanosheets with Ti3C2T MXene for enhancing water oxidation catalysis. Chem. Eng. J. 2020, 398, 125605. [Google Scholar] [CrossRef]
- Wen, Y. Synergistic cerium doping and MXene coupling in layered double hydroxides as efficient electrocatalysts for oxygen evolution. J. Energy Chem. 2021, 52, 412–420. [Google Scholar] [CrossRef]
- Yu, M. Boosting electrocatalytic oxygen evolution by synergistically coupling layered double hydroxide with MXene. Nano Energy 2018, 44, 181–190. [Google Scholar] [CrossRef]
- Zhang, C. Layer-by-layer assembly of exfoliated layered double hydroxide nanosheets for enhanced electrochemical oxidation of water. J. Mater. Chem. A 2016, 4, 11516–11523. [Google Scholar] [CrossRef]
- Zhao, L. Facile synthesis and efficient electrochemical water splitting of bifunctional nanostructured Ni-based layered double hydroxide/sulfide composite. J. Alloys Compd. 2022, 910, 164880. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Lu, Y.; Duanmu, M.; Zhao, X.; Song, S.; Duan, L.; Ma, Z.; Song, A.; Shao, G. Stably Improving the Catalytic Activity of Oxygen Evolution Reactions via Two-Dimensional Graphene Oxide-Incorporated NiFe-Layered Double Hydroxides. Catalysts 2024, 14, 278. https://doi.org/10.3390/catal14040278
Chen L, Lu Y, Duanmu M, Zhao X, Song S, Duan L, Ma Z, Song A, Shao G. Stably Improving the Catalytic Activity of Oxygen Evolution Reactions via Two-Dimensional Graphene Oxide-Incorporated NiFe-Layered Double Hydroxides. Catalysts. 2024; 14(4):278. https://doi.org/10.3390/catal14040278
Chicago/Turabian StyleChen, Ling, Yue Lu, Manman Duanmu, Xin Zhao, Shenglu Song, Liyue Duan, Zhipeng Ma, Ailing Song, and Guangjie Shao. 2024. "Stably Improving the Catalytic Activity of Oxygen Evolution Reactions via Two-Dimensional Graphene Oxide-Incorporated NiFe-Layered Double Hydroxides" Catalysts 14, no. 4: 278. https://doi.org/10.3390/catal14040278
APA StyleChen, L., Lu, Y., Duanmu, M., Zhao, X., Song, S., Duan, L., Ma, Z., Song, A., & Shao, G. (2024). Stably Improving the Catalytic Activity of Oxygen Evolution Reactions via Two-Dimensional Graphene Oxide-Incorporated NiFe-Layered Double Hydroxides. Catalysts, 14(4), 278. https://doi.org/10.3390/catal14040278