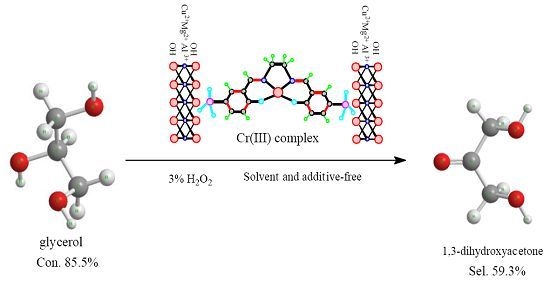
Selective Oxidation of Glycerol with 3% H2O2 Catalyzed by LDH-Hosted Cr(III) Complex
Abstract
:1. Introduction


2. Results and Discussion
2.1. Characterization of Catalysts
| Samples | Elemental Analysis Data (wt. %) | SBET (m2/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | Cr | Molar Ratio | C/N a | N/Cr b | ||
| MgAl-LDH | - | - | - | - | Mg/Al = 3/1 | - | - | 80.2 |
| NiAl-LDH | - | - | - | - | Ni/Al = 3/1 | - | - | 82.5 |
| ZnAl-LDH | - | - | - | - | Zn/Al = 3/1 | - | - | 81.7 |
| MgCr-LDH | - | - | - | - | Mg/Cr = 3/1 | - | - | 82.0 |
| MnMgAl-LDH | - | - | - | - | Mn/Mg/Al = 0.86/0.84/1.00 | - | - | 80.5 |
| CoMgAl-LDH | - | - | - | - | Co/Mg/Al = 0.85/0.84/1.00 | - | - | 78.9 |
| NiMgAl-LDH | - | - | - | - | Ni/Mg/Al = 0.86/0.85/1.00 | - | - | 79.5 |
| CuMgAl-LDH | - | - | - | - | Cu/Mg/Al = 0.88/0.88/1.00 | - | - | 80.2 |
| ZnMgAl-LDH | - | - | - | - | Zn/Mg/Al = 0.87/0.85/1.00 | - | - | 78.4 |
| Cr(SO3-salen) | 42.45 (42.46) c | 2.64 (2.65) | 6.17 (6.19) | 11.51 (11.50) | - | 8.03 (8.00) | 1.99 (2.00) | - |
| Cr(SO3-salen)-CuMgAl-LDH | 23.51 | 2.84 | 3.42 | 6.35 | Cu/Mg/Al = 0.87/0.88/1.00 | 8.02 (8.00) | 2.01 (2.00) | 66.3 |
| Cr(SO3-salen)-NiMgAl-LDH | 23.41 | 2.83 | 3.41 | 6.33 | Ni/Mg/Al = 0.87/0.86/1.00 | 8.01 (8.00) | 2.00 (2.00) | 65.5 |
| Cr(SO3-salen)-MnMgAl-LDH | 23.20 | 2.80 | 3.38 | 6.36 | Mn/Mg/Al = 0.86/0.85/1.00 | 8.00 (8.00) | 1.99 (2.00) | 65.8 |




2.2. Catalytic Performances of Catalysts
| Catalyst | GLY Con. (mol·%) | Sel. (mol·%) | |||||
|---|---|---|---|---|---|---|---|
| DHA | GLYAC | TARAC | GA | Oxalic Acid | Formic Acid | ||
| MgAl-LDH | 12.5 | 0 | 6.5 | 0 | 10.0 | 1.2 | 82.3 |
| NiAl-LDH | 4.5 | 0 | 3.0 | 2.5 | 13.5 | 1.5 | 79.5 |
| ZnAl-LDH | 5.0 | 0.3 | 4.5 | 1.6 | 17.6 | 2.2 | 73.8 |
| MgCr-LDH | 4.5 | 0.2 | 6.2 | 0.5 | 22.5 | 1.5 | 69.1 |
| MnMgAl-LDH | 14.2 | 0.2 | 10.7 | 1.5 | 20.6 | 2.0 | 65.0 |
| CoMgAl-LDH | 12.0 | 0 | 7.6 | 0 | 20.2 | 0 | 72.2 |
| NiMgAl-LDH | 25.5 | 0.5 | 19.5 | 3.7 | 15.0 | 1.4 | 59.9 |
| CuMgAl-LDH | 36.5 | 0 | 30.2 | 0 | 12.2 | 2.1 | 55.5 |
| ZnMgAl-LDH | 12.7 | 0 | 5.7 | 0 | 13.5 | 2.0 | 78.8 |
| Catalyst | GLY Con. (mol·%) | Sel. (mol·%) | |||||
|---|---|---|---|---|---|---|---|
| DHA | GLYAC | TARAC | GA | Oxalic Acid | Formic Acid | ||
| Cr(SO3-salen) | 38.2 | 11.5 | 13.9 | 0 | 0 | 0.3 | 74.3 |
| Cr(SO3-salen)-CuMgAl-LDH | 85.5 | 59.3 | 23.5 | 2.5 | 0 | 2.4 | 12.3 |
| Cr(SO3-salen)-NiMgAl-LDH | 82.1 | 61.2 | 28.5 | 0 | 0 | 0.3 | 10.0 |
| Cr(SO3-salen)-MnMgAl-LDH | 41.5 | 15.8 | 15.4 | 11.5 | 0.3 | 0 | 57.8 |



3. Experimental Section
3.1. Catalyst Preparation
3.1.1. Preparation of the Binary LDHs
3.1.2. Preparation of the Trinary LDHs
3.1.3. Preparation of the Heterogeneous LDH-Hosted Cr(III) Complex
3.2. Characterization
3.3. Catalytic Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Strelkova, T.V.; Mandelli, D. Oxidation of reactive alcohols with hydrogen peroxide catalyzed by manganese complexes. Catal. Lett. 2010, 138, 193–204. [Google Scholar] [CrossRef]
- Sankar, M.; Dimitratos, N.; Knight, D.W.; Carley, A.F.; Tiruvalam, R.; Kiely, C.J.; Thomas, D.; Hutchings, G.J. Oxidation of glycerol to glycolate by using supported gold and palladium nanoparticles. ChemSusChem 2009, 2, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Prati, L.; Spontoni, P.; Gaiassi, A. From renewable to fine chemicals through selective oxidation: The case of glycerol. Top. Catal. 2009, 52, 288–296. [Google Scholar] [CrossRef]
- Zhou, C.H.; Beltramini, J.N.; Lin, C.X.; Xu, Z.P.; Lu, G.Q.; Tanksale, A. Selective oxidation of biorenewable glycerol with molecular oxygen over Cu-containing layered double hydroxide-based catalysts. Catal. Sci. Technol. 2011, 1, 111–122. [Google Scholar] [CrossRef]
- Katryniok, B.; Kimura, J.; Skrzyńska, E.; Girardon, J.-S.; Fongarland, P.; Capron, M.; Ducoulombier, R.; Mimura, N.; Paul, S.; Dumeignil, F. Selective catalytic oxidation of glycerol: Perspectives for high value chemicals. Green Chem. 2011, 13, 1960–1979. [Google Scholar] [CrossRef]
- McMorn, P.; Roberts, G.; Hutchings, G.J. Oxidation of glycerol with hydrogen peroxide using silicalite and aluminophosphate catalysts. Catal. Lett. 1999, 63, 193–197. [Google Scholar] [CrossRef]
- Prati, L.; Villa, A.; Chan-Thaw, C.E.; Arrigo, R.; Wang, D.; Su, D.S. Gold catalyzed liquid phase oxidation of alcohol: The issue of selectivity. Faraday Discuss. 2011, 152, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A.; Rao, K.T.V.; Nishimura, S.; Takagaki, A.; Ebitani, K. Selective oxidation of glycerol by using a hydrotalcite-supported platinum catalyst under atmospheric oxygen pressure in water. ChemSusChem 2011, 4, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.F.; Shao, S.; Liu, C.L.; Xu, C.L.; Yang, R.Z.; Dong, W.S. Selective oxidation of glycerol over Pt supported on mesoporous carbon nitride in base-free aqueous solution. Chem. Eng. J. 2015, 264, 336–343. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Nie, R.F.; Wang, L.; Shi, J.J.; Du, W.C.; Hou, Z.Y. Selective oxidation of glycerol over carbon nanofibers supported Pt catalysts in a base-free aqueous solution. Catal. Commun. 2015, 59, 5–9. [Google Scholar] [CrossRef]
- Zope, B.N.; Hibbitts, D.D.; Neurock, M.; Davis, R.J. Reactivity of the gold/water interface during selective oxidation catalysis. Science 2010, 330, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Kapkowski, M.; Bartczak, P.; Korzec, M.; Sitko, R.; Szade, J.; Balin, K.; Lelatko, J.; Polanski, J. SiO2-, Cu-, and Ni-supported Au nanoparticles for selective glycerol oxidation in the liquid phase. J. Catal. 2014, 319, 110–118. [Google Scholar] [CrossRef]
- Skrzyńska, E.; Ftouni, J.; Mamede, A.S.; Addad, A.; Trentesaux, M.; Girardon, J.S.; Capron, M.; Dumeignil, F. Glycerol oxidation over gold supported catalysts–“Two faces” of sulphur based anchoring agent. J. Mol. Catal. A 2014, 382, 71–78. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Portilho, M.F.; Silva, A.C.; Taroco, H.A.; Souza, P.P. Modified niobia as a bifunctional catalyst for simultaneous dehydration and oxidation of glycerol. Appl. Catal. B 2012, 117–118, 29–35. [Google Scholar] [CrossRef]
- Wang, X.L.; Wu, G.D.; Wang, F.; Ding, K.Q.; Zhang, F.; Liu, X.F.; Xue, Y.B. Base-free selective oxidation of glycerol with 3% H2O2 catalyzed by sulphonato-salen-chromium (III) intercalated LDH. Catal. Commun. 2012, 28, 73–76. [Google Scholar] [CrossRef]
- Crotti, C.; Farnetti, E. Selective oxidation of glycerol catalyzed by iron complexes. J. Mol. Catal. A 2015, 396, 353–359. [Google Scholar] [CrossRef]
- Oliveira, V.L.; Morais, C.; Servat, K.; Napporn, T.W.; Tremiliosi-Filho, G.; Kokoh, K.B. Glycerol oxidation on nickel based nanocatalysts in alkaline medium–Identification of the reaction products. J. Electroanal. Chem. 2013, 703, 56–62. [Google Scholar] [CrossRef]
- Wu, G.D.; Wang, X.L.; Li, J.P.; Zhao, N.; Wei, W.; Sun, Y.H. A new route to synthesis of sulphonato-salen-chromium (III) hydrotalcites: Highly selective catalysts for oxidation of benzyl alcohol to benzaldehyde. Catal. Today 2008, 131, 402–407. [Google Scholar] [CrossRef]
- Srinivasan, K.; Kochi, J.K. Synthesis and molecular structure of oxochromium (V) cations. coordination with donor ligands. Inorg. Chem. 1985, 24, 4671–4679. [Google Scholar] [CrossRef]
- Choudary, B.M.; Ramani, T.; Maheswaran, H.; Prashant, L.; Ranganath, K.V.S.; Kumarb, K.V. Catalytic asymmetric epoxidation of unfunctionalised olefins using silica, LDH and resin-supported sulfonato-Mn (salen) complex. Adv. Synth. Catal. 2006, 348, 493–498. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Anderson, J.A. Comparison of the epoxidation of cyclohexene, dicyclopentadiene and 1,5-cyclooctadiene over LDH hosted Fe and Mn sulfonato-salen complexes. J. Mol. Catal. A 2006, 249, 103–110. [Google Scholar] [CrossRef]
- Kingma, I.E.; Wiersma, M.; van der Baan, J.L.; Balt, S.; Bickelheanpt, F.; de Bolster, M.W.G.; Klumpp, G.W.; Spek, A.L. Intramolecular alkoxycobaltation: A novel route to the cobalt-carbon bond in a coenzyme B12 model. Chem. Commun. 1993. [Google Scholar] [CrossRef]
- Mukherjee, S.; Samanta, S.; Roy, B.C.; Bhaumik, A. Efficient allylic oxidation of cyclohexene catalyzed by immobilized Schiff base complex using peroxides as oxidants. Appl. Catal. A 2006, 301, 79–88. [Google Scholar] [CrossRef]
- Cantrell, D.G.; Gillie, L.J.; Lee, A.F.; Wilson, K. Structure-reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis. Appl. Catal. A 2005, 287, 183–190. [Google Scholar] [CrossRef]
- Zhou, H.; Zhuo, G.L.; Jiang, X.Z. Heck reaction catalyzed by Pd supported on LDH-F hydrotalcite. J. Mol. Catal. A 2006, 248, 26–31. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, G.; Wang, X.; Jiang, T.; Lin, Q. Selective Oxidation of Glycerol with 3% H2O2 Catalyzed by LDH-Hosted Cr(III) Complex. Catalysts 2015, 5, 2039-2051. https://doi.org/10.3390/catal5042039
Wu G, Wang X, Jiang T, Lin Q. Selective Oxidation of Glycerol with 3% H2O2 Catalyzed by LDH-Hosted Cr(III) Complex. Catalysts. 2015; 5(4):2039-2051. https://doi.org/10.3390/catal5042039
Chicago/Turabian StyleWu, Gongde, Xiaoli Wang, Taineng Jiang, and Qibo Lin. 2015. "Selective Oxidation of Glycerol with 3% H2O2 Catalyzed by LDH-Hosted Cr(III) Complex" Catalysts 5, no. 4: 2039-2051. https://doi.org/10.3390/catal5042039
APA StyleWu, G., Wang, X., Jiang, T., & Lin, Q. (2015). Selective Oxidation of Glycerol with 3% H2O2 Catalyzed by LDH-Hosted Cr(III) Complex. Catalysts, 5(4), 2039-2051. https://doi.org/10.3390/catal5042039