A Microwave-Sensitive Solid Acid Catalyst Prepared from Sweet Potato via a Simple Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microwave Sensitive and Homogeneous Properties
2.2. Catalytic Activity
2.2.1. Effects of Microwave Power Density
2.2.2. Effect of Moisture Content under Microwave Heating Environment
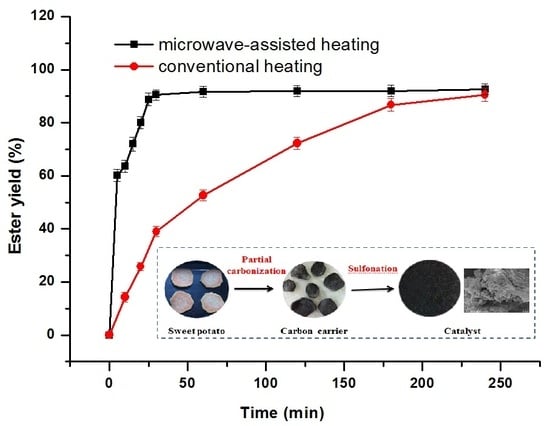
2.2.3. Comparison of Catalyst Type and Reaction-Heating Method
2.2.4. Effect of Reusability and Regeneration
2.3. Catalyst Physicochemical Properties Characterization
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Investigation of Microwave-Sensitive and Homogeneous Properties
3.4. Catalytic Esterification Activity Measurements
3.5. Physicochemical Properties Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chai, F.; Cao, F.H.; Zhai, F.Y.; Chen, Y.; Wang, X.H.; Su, Z.M. Transesterification of vegetable oil to biodiesel using a heteropolyacid solid catalyst. Adv. Synth. Catal. 2007, 349, 1057–1065. [Google Scholar] [CrossRef]
- Kiss, A.A.; Dimian, A.C.; Rothenberg, G. Solid acid catalysts for biodiesel production-towards sustainable energy. Adv. Synth. Catal. 2006, 348, 75–81. [Google Scholar] [CrossRef]
- Lotero, E.; Liu, Y.J.; Lopez, D.E.; Suwannakarn, K.; Bruce, D.A.; Goodwin, J.G. Synthesis of biodiesel via acid catalysis. Ind. Eng. Chem. Res. 2005, 44, 5353–5363. [Google Scholar] [CrossRef]
- Takagaki, A.; Toda, M.; Okamura, M.; Kondo, J.N.; Hayashi, S.; Domen, K.; Hara, M. Esterrification of higher fatty acids by a novel strong solid acid. Catal. Today 2006, 116, 157–161. [Google Scholar] [CrossRef]
- Vicente, G.; Martinez, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Fang, B. Preparation of solid acid catalyst from glucose-starch mixture for biodiesel production. Bioresour. Technol. 2011, 102, 2635–2640. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Takagak, A.; Okatnura, M.; Kondo, J.N.; Hayashi, S.; Domen, K.; Hara, M. Green chemistry—Biodiesel made with sugar catalyst. Nature 2005, 438, 178. [Google Scholar] [CrossRef] [PubMed]
- Zong, M.H.; Duan, Z.Q.; Lou, W.Y.; Thomas, J.S.; Wu, H. Preparation of a sugar catalyst and its use for highly efficient production of biodiesel. Green Chem. 2007, 9, 434–437. [Google Scholar] [CrossRef]
- Su, F.; Guo, Y.H. Advancements in solid acid catalysts for biodiesel production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef] [PubMed]
- Hemando, J.; Leton, P.; Matia, M.P.; Novella, J.L.; Alvarez-Builla, J. Biodiesel and FAME synthesis assisted by microwaves: Homogeneous batch and flow processes. Fuel 2007, 86, 1641–1644. [Google Scholar] [Green Version]
- Leadbeater, N.E.; Stencel, L.M. Fast, easy, preparation of biodiesel using microwave heating. Energy Fuel 2006, 20, 2281–2283. [Google Scholar] [CrossRef]
- Motasemi, F.; Ani, F.N. A review on microwave-assisted production of biodiesel. Renew. Sustain. Energy Rev. 2012, 16, 4719–4733. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, B.L.; Zhu, G.L. Synthesis of biodiesel using microwave absorption catalysts. Energy Fuel 2009, 23, 548–552. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, S.; Gong, W.; Wang, G.; Qiu, J.; Chen, H. Synthesis, characterization and acid catalysis of solid acid from peanut shell. Appl. Catal. A 2014, 469, 284–289. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Waje, M.M.; Chen, Z.; Yan, Y.; Bozhilov, K.N.; Feng, P. Sulfonated ordered mesoporous carbon as a stable and highly active protonic acid catalyst. Chem. Mater. 2007, 19, 2395–2397. [Google Scholar] [CrossRef]
- Zhang, S.; Zu, Y.G.; Fu, Y.J.; Luo, M.; Zhang, D.Y.; Efferth, T. Rapid microwave-assisted transesterification of yellow horn oil to biodiesel using a heterpolyacid solid catalyst. Bioresour. Technol. 2011, 101, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Lou, W.Y.; Zong, M.H.; Duan, Z.Q. Efficient production of biodiesel from high free fatty acid-containing waste oils using various carbohydrate-derived solid acid catalysts. Bioresour. Technol. 2008, 99, 8752–8758. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Huang, M.; Ma, H.L.; Zhang, Z.Q.; Gao, J.M.; Zhu, Y.L.; Han, X.J.; Guo, X.Y. Preparation of a carbon-based acid catalyst by sulfonating activated carbon in a chemical reduction process. Molecules 2010, 15, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.W.; Wu, W.W.; Wang, S.S.; Shi, X.Y.; Wu, F.A.; Wang, J. Preparation and characterization of a solid acid catalyst from macro Fungi residue for methyl palmitate production. BioResources 2015, 10, 5691–5708. [Google Scholar] [CrossRef]
- Liu, T.T.; Li, Z.L.; Li, W.; Shi, C.J.; Wang, Y. Preparation and characterization of biomass carbon-based solid acid catalyst for the esterification of oleic acid with methanol. Bioresour. Technol. 2013, 133, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Liu, H.; Li, L. Carbon-based acid catalyst from waste seed shells: Preparation and characterization. Pol. J. Chem. Technol. 2015, 17, 37–41. [Google Scholar] [CrossRef]
- Zeng, D.L.; Zhang, Q.; Chen, S.Y.; Liu, S.L.; Wang, G.H. Synthesis porous carbon-based solid acid from rice husk for esterification of fatty acids. Microporous Mesoporous Mater. 2016, 219, 54–58. [Google Scholar] [CrossRef]
- Geng, L.; Wang, Y.; Yu, G.; Zhu, Y.X. Efficient carbon-based solid acid catalysts for the esterification of oleic acid. Catal. Commun. 2011, 13, 26–30. [Google Scholar] [CrossRef]
- Mo, X.; Lopez, D.E.; Suwannakarn, K.; Liu, Y.; Lotero, E.; Goodwin, J.G.; Lu, C.Q. Activation and deactivation characteristics of sulfonated carbon catalysts. J. Catal. 2008, 254, 332–338. [Google Scholar] [CrossRef]
- Suppalakpanya, K.; Ratanawilai, S.B.; Tongurai, C. Production of ethylester from esterified crude palm oil by microwave with dry washing by bleaching earth. Appl. Energy 2010, 87, 2356–2359. [Google Scholar] [CrossRef]
- Tsubouchi, N.; Xu, C.B.; Ohtsuka, Y. Carbon crystallization during high-temperature pyrolysis of coals and the enhancement by calcium. Energy Fuel 2003, 17, 1119–1125. [Google Scholar] [CrossRef]
- Liang, X.Z.; Yang, J.G. Sythesis of a novel carbon based strong acid catalyst through hydrothermal carbonization. Catal. Lett. 2009, 132, 460–463. [Google Scholar] [CrossRef]
- Soares, S.; Camino, G. Comparative study of the thermal decomposition of pure cellulose and pulp paper. Polym. Degrad. Stab. 1995, 49, 275–283. [Google Scholar] [CrossRef]
- Devi, B.A.P.; Gangadhar, K.N.; Prasad, P.S.P.; Jagannadh, B.; Prasad, R.B.N. A glycerol-based carbon catalyst for the preparation of biodiesel. ChemSusChem 2009, 2, 617–620. [Google Scholar] [CrossRef] [PubMed]








| Heating Rate | 100 W | 200 W | 300 W | |||
|---|---|---|---|---|---|---|
| A (°C/min) | B (°C/min) | A (°C/min) | B (°C/min) | A (°C/min) | B (°C/min) | |
| k | 0.91 | 3.00 | 2.03 | 6.58 | 4.50 | 8.79 |
| Catalyst Material | Molar Ratio of Alcohol and Fatty Acid | Activity | Ref. |
|---|---|---|---|
| Sweet potatoes | methanol/oleic acid 6:1 | 91% | This study |
| Glucose–starch mixture | methanol/oleic acid 10:1 | 96% | [6] |
| d-glucose | methanol/oleic acid 10:1 | 95% | [8] |
| Peanut shell | methanol/cottonseed oil 9:1 | 90.2% | [15] |
| Starch | methanol/oleic acid 10:1 | 95% | [18] |
| Cellulose | methanol/oleic acid 10:1 | 88% | [18] |
| Activated carbon | ethanol/acetic acid 10:1 | 78% | [19] |
| Phellinus igniarius | methanol/palmitate acid 10:1 | 91.5% | [20] |
| Corn straw | methanol/oleic acid 7:1 | 98% | [21] |
| Seed shells | methanol/oleic acid 1:1 | 95.7% | [22] |
| Rice husk | methanol/oleic acid 5:1 | 91% | [23] |
| Runs | Ester Yield (%) | |
|---|---|---|
| Sulfonated Carbon-Based Solid Catalyst | Regenerated Carbon-Based Solid Catalyst | |
| 1 | 91.12 | 87.56 |
| 2 | 87.32 | 81.35 |
| 3 | 81.05 | 76.07 |
| 4 | 75.64 | 68.38 |
| 5 | 64.38 | 56.25 |
| Sample | SBET (m2/g) | Vtot (cm3/g) | D (nm) | S Content (%) | Total Acid Density (mmol/g) |
|---|---|---|---|---|---|
| Solid acid (fresh) | 78.35 | 0.634 | 42.86 | 7.449 | 6.35 |
| Solid acid (spent) | 43.45 | 0.598 | 45.36 | 5.152 | 4.39 |
| Solid acid (regenerated) | 67.32 | 0.602 | 40.28 | 7.158 | 6.10 |
| Samples | C% | H% | O% | S% |
|---|---|---|---|---|
| Sweet potato | 40.46 | 6.484 | 52.329 | 0 |
| Carbonization carrier | 69.80 | 4.052 | 24.859 | 0 |
| Sulfonated carbon-based catalyst | 47.88 | 4.471 | 39.376 | 7.449 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-Y.; Cui, Z.-W. A Microwave-Sensitive Solid Acid Catalyst Prepared from Sweet Potato via a Simple Method. Catalysts 2016, 6, 211. https://doi.org/10.3390/catal6120211
Chen H-Y, Cui Z-W. A Microwave-Sensitive Solid Acid Catalyst Prepared from Sweet Potato via a Simple Method. Catalysts. 2016; 6(12):211. https://doi.org/10.3390/catal6120211
Chicago/Turabian StyleChen, Hai-Ying, and Zheng-Wei Cui. 2016. "A Microwave-Sensitive Solid Acid Catalyst Prepared from Sweet Potato via a Simple Method" Catalysts 6, no. 12: 211. https://doi.org/10.3390/catal6120211
APA StyleChen, H. -Y., & Cui, Z. -W. (2016). A Microwave-Sensitive Solid Acid Catalyst Prepared from Sweet Potato via a Simple Method. Catalysts, 6(12), 211. https://doi.org/10.3390/catal6120211