Base-Free Selective Oxidation of Glycerol over LDH Hosted Transition Metal Complexes Using 3% H2O2 as Oxidant
Abstract
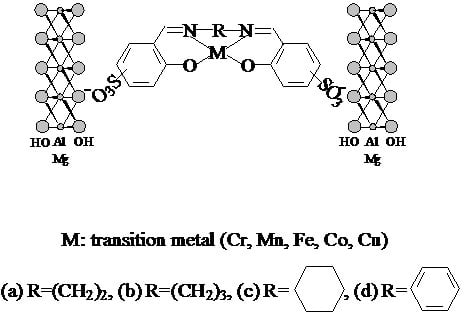
:1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
2.2. Catalytic Performance
3. Experimental Section
3.1. Catalyst Preparation
3.2. Characterization
3.3. Catalytic Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, C.H.; Beltramini, J.N.; Lin, C.X.; Xu, Z.P.; Lu, G.Q.; Tanksale, A. Selective oxidation of biorenewable glycerol with molecular oxygen over Cu-containing layered double hydroxide-based catalysts. Catal. Sci. Technol. 2011, 1, 111–122. [Google Scholar] [CrossRef]
- Brett, G.L.; He, Q.; Hammond, C.; Miedziak, P.J.; Dimitratos, N.; Sankar, M.; Herzing, A.A.; Conte, M.; Lopez-Sanchez, J.A.; Kiely, C.J.; et al. Selective oxidation of glycerol by highly active bimetallic catalysts at ambient temperature under base-free conditions. Angew. Chem. Int. Ed. 2011, 50, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.Z.; Davis, R.J. Glycerol-intercalated Mg-Al hydrotalcite as a potential solid base catalyst for transesterification. Clays Clay Miner. 2010, 58, 475–485. [Google Scholar]
- Zhou, C.H.; Beltramini, J.N.; Fan, Y.X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Prati, L.; Villa, A.; Chan-Thaw, C.E.; Arrigo, R.; Wang, D.; Su, D.S. Gold catalyzed liquid phase oxidation of alcohol: The issue of selectivity. Faraday Discuss. 2011, 152, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A.; Rao, K.T.V.; Nishimura, S.; Takagaki, A.; Ebitani, K. Selective oxidation of glycerol by using a hydrotalcite-supported platinum catalyst under atmospheric oxygen pressure in water. ChemSusChem 2011, 4, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.F.; Shao, S.; Liu, C.L.; Xu, C.L.; Yang, R.Z.; Dong, W.S. Selective oxidation of glycerol over Pt supported on mesoporous carbon nitride in base-free aqueous solution. Chem. Eng. J. 2015, 264, 336–343. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Nie, R.F.; Wang, L.; Shi, J.J.; Du, W.C.; Hou, Z.Y. Selective oxidation of glycerol over carbon nanofibers supported Pt catalysts in a base-free aqueous solution. Catal. Commun. 2015, 59, 5–9. [Google Scholar] [CrossRef]
- Zope, B.N.; Hibbitts, D.D.; Neurock, M.; Davis, R.J. Reactivity of the gold/water interface during selective oxidation catalysis. Science 2010, 330, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Sankar, M.; Dimitratos, N.; Knight, D.W.; Carley, A.F.; Tiruvalam, R.; Kiely, C.J.; Thomas, D.; Hutchings, G.J. Oxidation of glycerol to glycolate by using supported gold and palladium nanoparticles. ChemSusChem 2009, 2, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Kapkowski, M.; Bartczak, P.; Korzec, M.; Sitko, R.; Szade, J.; Balin, K.; Lelątko, J.; Polanski, J. SiO2-, Cu-, and Ni-supported Au nanoparticles for selective glycerol oxidation in the liquid phase. J. Catal. 2014, 319, 110–118. [Google Scholar] [CrossRef]
- Rodrigues, E.G.; Pereira, F.R.; Chen, X.W.; Delgado, J.J.; Orfao, J.J.M. Selective oxidation of glycerol over platinm-based catalysts supported on carbon nanotubes. Ind. Eng. Chem. Res. 2013, 52, 17390–17398. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Strelkova, T.V.; Mandelli, D. Oxidation of reactive alcohols with hydrogen peroxide catalyzed by manganese complexes. Catal. Lett. 2010, 138, 193–204. [Google Scholar] [CrossRef]
- McMorn, P.; Roberts, G.; Hutchings, G.J. Oxidation of glycerol with hydrogen peroxide using silicalite and aluminophosphate catalysts. Catal. Lett. 1999, 63, 193–197. [Google Scholar] [CrossRef]
- Savanur, A.P.; Nandibewoor, S.T.; Chimatadar, S.A. Manganese (II) catalysed oxidation of glycerol by cerium (IV) in aqueous sulphuric acid medium: a kinetic and mechanistic study. Transition Met. Chem. 2009, 34, 711–718. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Portilho, M.F.; Silva, A.C.; Taroco, H.A.; Souza, P.P. Modified niobia as a bifunctional catalyst for simultaneous dehydration and oxidation of glycerol. Appl. Catal. B Envioron. 2012, 117, 29–35. [Google Scholar] [CrossRef]
- Wang, X.L.; Wu, G.D.; Wang, F.; Ding, K.Q.; Zhang, F.; Liu, X.F.; Xue, Y.B. Base-free selective oxidation of glycerol with 3% H2O2 catalyzed by sulphonato-salen-chromium (III) intercalated LDH. Catal. Commun. 2012, 28, 73–76. [Google Scholar] [CrossRef]
- Crotti, C.; Farnetti, E. Selective oxidation of glycerol catalyzed by iron complexes. J. Mol. Catal. A Chem. 2015, 396, 353–359. [Google Scholar] [CrossRef]
- Oliveira, V.L.; Morais, C.; Servat, K.; Napporn, T.W.; Tremiliosi-Filho, G.; Kokoh, K.B. Glycerol oxidation on nickel based nanocatalysts in alkaline medium–Identification of the reaction products. J. Electroanal. Chem. 2013, 703, 56–62. [Google Scholar] [CrossRef]
- Wu, G.D.; Wang, X.L.; Huang, Y.A.; Liu, X.F.; Zhang, F.; Ding, K.Q.; Yang, X.L. Selective oxidation of glycerol with O2 catalyzed by low-cost CuNiAl hydrotalcites. J. Mol. Catal. A Chem. 2013, 379, 185–191. [Google Scholar] [CrossRef]
- Urdă, A.; Popescu, I.; Cacciaguerra, T.; Tanchoux, N.; Tichit, D.; Marcu, I.C. Total oxidation of methane over rare earth cation-containing mixed oxides derived from LDH precursors. Appl. Catal. A Gen. 2013, 464–465, 20–27. [Google Scholar] [CrossRef]
- Dobrosza, I.; Jiratovab, K.; Pitchon, V.; Rynkowski, J.M. Effect of the preparation of supported gold particles on the catalytic activity in CO oxidation reaction. J. Mol. Catal. A Chem. 2005, 234, 187–197. [Google Scholar] [CrossRef]
- Varga, G.; Ziegenheim, S.; Muráth, S.; Csendes, Z.; Kukovecz, A.; Kónya, Z.; Carlson, S.; Korecz, L.; Varga, E.; Pusztai, P.; et al. Cu(II)-amino acid–CaAl-layered double hydroxide complexes, recyclable, efficient catalysts in various oxidative transformations. J. Mol. Catal. A Chem. 2016, 423, 49–60. [Google Scholar] [CrossRef]
- Lv, W.M.; Yang, L.; Fan, B.B.; Zhao, Y.; Chen, Y.F.; Lu, N.Y.; Li, R.F. Silylated MgAl LDHs intercalated with MnO2 nanowires: Highly efficient catalysts for the solvent-free aerobic oxidationof ethylbenzene. Chem. Eng. J. 2015, 263, 309–316. [Google Scholar] [CrossRef]
- Wu, G.D.; Wang, X.L.; Li, J.P.; Zhao, N.; Wei, W.; Sun, Y.H. A new route to synthesis of sulphonato-salen-chromium (III) hydrotalcites: Highly selective catalysts for oxidation of benzyl alcohol to benzaldehyde. Catal. Today 2008, 131, 402–407. [Google Scholar] [CrossRef]
- Choudary, B.M.; Ramani, T.; Maheswaran, H.; Prashant, L.; Ranganath, K.V.S.; Kumarb, K.V. Catalytic asymmetric epoxidation of unfunctionalised olefins using silica, LDH and resin-supported sulfonato-Mn (salen) complex. Adv. Synth. Catal. 2006, 348, 493–498. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Anderson, J.A. Comparison of the epoxidation of cyclohexene, dicyclopentadiene and 1,5-cyclooctadiene over LDH hosted Fe and Mn sulfonato-salen complexes. J. Mol. Catal. A Chem. 2006, 249, 103–110. [Google Scholar] [CrossRef]
- Cantrell, D.G.; Gillie, L.J.; Lee, A.F.; Wilson, K. Structure-reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis. Appl. Catal. A Gen. 2005, 287, 183–190. [Google Scholar] [CrossRef]
- Zhou, H.; Zhuo, G.L.; Jiang, X.Z. Heck reaction catalyzed by Pd supported on LDH-F hydrotalcite. J. Mol. Catal. A Chem. 2006, 248, 26–31. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Karandikar, P.; Dhanya, K.C.; Deshpande, S.; Chandwadkar, A.J.; Sivasanker, S.; Agashe, M. Cu/Co-salen immobilized MCM-41: characterization and catalytic reactions. Catal. Commun. 2004, 5, 69–74. [Google Scholar] [CrossRef]








| Samples | Elemental Analysis Data (wt %) | SBET (m2g−1) | FTIR Data (cm–1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | Mg | Al | M a | N/M b | |||
| LDH-[C6H5COO] | 20.24 | 4.23 | 39.65 | - | - | 20.36 | 6.52 | - | - | 80.2 | - |
| LDH-[Cr(SO3-salen)] | 25.62 | 3.17 | 36.71 | 3.44 | 7.83 | 12.66 | 4.21 | 6.36 | 2.01 (2.00)c | 66.5k | 1621, 1529, 1112, 1034, 597, 418 |
| LDH-[Cr(SO3-salan)] | 24.79 | 3.14 | 40.64 | 3.40 | 7.78 | 10.53 | 3.40 | 6.32 | 1.99 (2.00) | 64.2 | 1620, 1522, 1110, 1035, 611, 476 |
| LDH-[Cr(SO3-sahen)] | 29.03 | 3.25 | 36.80 | 3.38 | 7.74 | 10.19 | 3.32 | 6.29 | 2.00 (2.00) | 59.6 | 1620, 1519, 1112, 1035, 602, 535 |
| LDH-[Cr(SO3-salphen)] | 29.07 | 3.41 | 36.36 | 3.39 | 7.75 | 10.25 | 3.47 | 6.30 | 1.99 (2.00) | 57.5 | 1624, 1520, 1114, 1039, 605, 412 |
| LDH-[Mn(SO3-salphen)] | 26.52 | 3.16 | 39.17 | 3.10 | 7.07 | 11.34 | 3.55 | 6.09 | 2.00 (2.00) | 60.2 | 1625, 1520, 1115, 1035, 602, 418 |
| LDH-[Fe(SO3-salphen)] | 24.06 | 3.20 | 42.14 | 2.81 | 6.41 | 12.20 | 3.62 | 5.56 | 2.02 (2.00) | 59.0 | 1620, 1518, 1110, 1035, 601, 417 |
| LDH-[Co(SO3-salphen)] | 21.32 | 3.12 | 44.73 | 2.47 | 5.68 | 13.45 | 3.85 | 5.18 | 2.01 (2.00) | 59.7 | 1622, 1519, 1112, 1036, 600, 420 |
| LDH-[Cu(SO3-salphen)] | 20.02 | 3.08 | 46.26 | 2.33 | 5.22 | 13.71 | 4.05 | 5.33 | 2.00 (2.00) | 58.5 | 1624, 1520, 1115, 1040, 610, 415 |
| Entry | Catalysts | M b (wt %) | GLY Con. (mol%) | Sel. (mol%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| DHA | Glyceric Acid | Tartronic Acid | Hydroxypyruvic Acid | Formic Acid | Oxalic Acid | ||||
| 1 | Blank | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | LDH | - | 6.4 | 0 | 1.6 | 0.2 | 0 | 92.6 | 5.6 |
| 3 | Cr(SO3-salen) | 11.50 | 36.1 | 9.5 | 13.7 | 0.8 | 0 | 75.8 | 0.2 |
| 4 | Cr(SO3-salan) | 11.15 | 35.5 | 10.2 | 13.6 | 1.2 | 0 | 69.8 | 5.2 |
| 5 | Cr(SO3-sahen) | 10.23 | 30.2 | 10.5 | 7.3 | 1.6 | 5.5 | 70.5 | 4.6 |
| 6 | Cr(SO3-salphen) | 10.35 | 38.5 | 7.9 | 11.8 | 2.5 | 2.3 | 73.6 | 1.9 |
| 7 | LDH-[Cr(SO3-salen)] | 6.36 | 71.3 | 43.5 | 20.9 | 0 | 0 | 35.2 | 0.4 |
| 8 | LDH-[Cr(SO3-salan)] | 6.32 | 69.4 | 45.7 | 12.0 | 2.0 | 2.5 | 32.1 | 5.7 |
| 9 | LDH-[Cr(SO3-sahen)] | 6.29 | 48.0 | 22.1 | 14.2 | 3.5 | 12.0 | 38.0 | 10.2 |
| 10 | LDH-[Cr(SO3-salphen)] | 5.65 | 60.0 | 38.6 | 12.3 | 3.2 | 9.5 | 29.6 | 6.8 |
| 11 | 6.05 | 75.2 | 46.5 | 13.5 | 2.9 | 6.7 | 28.9 | 7.5 | |
| 12 | 6.30 | 80.8 | 52.5 | 11.6 | 1.9 | 3.8 | 23.7 | 6.5 | |
| 13 | 6.75 | 57.5 | 40.1 | 11.5 | 2.2 | 5.6 | 31.4 | 9.2 | |
| 14 | LDH-[Mn(SO3-salphen)] | 6.29 | 29.4 | 3.7 | 0.9 | 7.0 | 25.5 | 12.1 | 50.8 |
| 15 | LDH-[Fe(SO3-salphen)] | 6.30 | 45.0 | 38.1 | 9.2 | 6.5 | 20.4 | 10.4 | 15.4 |
| 16 | LDH-[Co(SO3-salphen)] | 6.30 | 63.8 | 40.4 | 10.6 | 1.9 | 3.8 | 19.8 | 24.5 |
| 17 | LDH-[Cu(SO3-salphen)] | 6.35 | 58.0 | 41.5 | 9.5 | 2.2 | 5.0 | 19.5 | 22.3 |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Shang, C.; Wu, G.; Liu, X.; Liu, H. Base-Free Selective Oxidation of Glycerol over LDH Hosted Transition Metal Complexes Using 3% H2O2 as Oxidant. Catalysts 2016, 6, 101. https://doi.org/10.3390/catal6070101
Wang X, Shang C, Wu G, Liu X, Liu H. Base-Free Selective Oxidation of Glycerol over LDH Hosted Transition Metal Complexes Using 3% H2O2 as Oxidant. Catalysts. 2016; 6(7):101. https://doi.org/10.3390/catal6070101
Chicago/Turabian StyleWang, Xiaoli, Congxiao Shang, Gongde Wu, Xianfeng Liu, and Hao Liu. 2016. "Base-Free Selective Oxidation of Glycerol over LDH Hosted Transition Metal Complexes Using 3% H2O2 as Oxidant" Catalysts 6, no. 7: 101. https://doi.org/10.3390/catal6070101
APA StyleWang, X., Shang, C., Wu, G., Liu, X., & Liu, H. (2016). Base-Free Selective Oxidation of Glycerol over LDH Hosted Transition Metal Complexes Using 3% H2O2 as Oxidant. Catalysts, 6(7), 101. https://doi.org/10.3390/catal6070101