Highly Efficient Tetranuclear ZnII2LnIII2 Catalysts for the Friedel–Crafts Alkylation of Indoles and Nitrostyrenes
Abstract
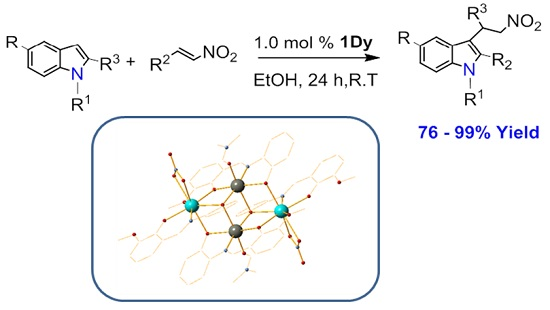
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Synthesis of Catalysts
4.2. General Procedure for the Friedel–Craft Reaction
4.3. Single Crystal X-Ray Structure Determinations
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Olah, G.A.; Khrisnamurti, R.; Prakash, G.K.S.I. Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon: Oxford, UK, 1991. [Google Scholar]
- Roberts, R.M.; Khalaf, A.A. Friedel–Crafts Alkylation Chemistry: A Century of Discovery; Marcel, D., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1984. [Google Scholar]
- Sundberg, R. The Chemistry of Indoles; Academic: New York, NY, USA, 1996. [Google Scholar]
- Lancianesi, S.; Palmieri, A.; Petrini, M. Synthetic Approaches to 3-(2-Nitroalkyl) Indoles and Their Use to Access Tryptamines and Related Bioactive Compounds. Chem. Rev. 2014, 114, 7108–7149. [Google Scholar] [CrossRef] [PubMed]
- Berner, O.M.; Tedeschi, L.; Enders, D. Asymmetric Michael additions to nitroalkenes. Eur. J. Org. Chem. 2002, 2002, 1877–1894. [Google Scholar] [CrossRef]
- Ono, N. Synthesis of Heterocyclic Compounds. In The Nitro Group in Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Papadas, I.T.; Fountoulaki, S.; Lykakis, I.N.; Armatas, G.S. Controllable Synthesis of Mesoporous Iron Oxide Nanoparticle Assemblies for Chemoselective Catalytic Reduction of Nitroarenes. Chem. Eur. J. 2016, 22, 4600–4607. [Google Scholar] [CrossRef] [PubMed]
- Fountoulaki, S.; Daikopoulou, V.; Gkizis, P.L.; Tamiolakis, I.; Armatas, G.S.; Lykakis, I.N. Mechanistic studies of the reduction of nitroarenes by NaBH4 or hydrosilanes catalyzed by supported gold nanoparticles. ACS Catal. 2014, 4, 3504–3511. [Google Scholar] [CrossRef]
- Stratakis, M.; Garcia, H. Catalysis by supported gold nanoparticles: Beyond aerobic oxidative processes. Chem. Rev. 2012, 112, 4469–4506. [Google Scholar] [CrossRef] [PubMed]
- Blaser, H.-U.; Steiner, H.; Studer, M. Selective catalytic hydrogenation of functionalized nitroarenes: An update. ChemCatChem 2009, 1, 210–221. [Google Scholar] [CrossRef]
- Herrera, R.P.; Sgarzani, V.; Bernardi, L.; Ricci, A. Catalytic enantioselective Friedel–Crafts alkylation of indoles with nitroalkenes by using a simple thiourea organocatalyst. Angew. Chem. Int. Ed. 2005, 44, 6576–6579. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Hazell, R.G.; Jørgensen, K.A. Enantioselective Friedel–Crafts type addition of indoles to nitro-olefins using a chiral hydrogen-bonding catalyst-synthesis of optically active tetrahydro-β-carbolines. Org. Biomol. Chem. 2005, 3, 2566–2571. [Google Scholar] [CrossRef] [PubMed]
- Fleming, E.M.; McCabe, T.; Connon, S.J. Novel axially chiral bis-arylthiourea-based organocatalysts for asymmetric Friedel–Crafts type reactions. Tetrahedron Lett. 2006, 47, 7037–7042. [Google Scholar] [CrossRef]
- Li, L.; Ganesh, M.; Seidel, D. Catalytic Enantioselective Synthesis of α,β-Diamino Acid Derivatives. J. Am. Chem. Soc. 2009, 130, 16464–16465. [Google Scholar] [CrossRef] [PubMed]
- Marqués-López, E.; Alcaine, A.; Tejero, T.; Herrera, R.P. Enhanced Efficiency of Thiourea Catalysts by External Brønsted Acids in the Friedel–Crafts Alkylation of Indoles. Eur. J. Org. Chem. 2011, 2011, 3700–3705. [Google Scholar] [CrossRef] [Green Version]
- Itoh, J.; Fuchibe, K.; Akiyama, T. Chiral phosphoric acid catalyzed enantioselective Friedel–Crafts alkylation of indoles with nitroalkenes: Cooperative effect of 3 Å molecular sieves. Angew. Chem. Int. Ed. 2008, 47, 4016–4018. [Google Scholar] [CrossRef] [PubMed]
- So, S.S.; Burkett, J.A.; Mattson, A.E. Internal Lewis acid assisted hydrogen bond donor catalysis. Org. Lett. 2011, 13, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Roca-Lopez, D.; Marques-Lopez, E.; Alcaine, A.; Merino, P.; Herrera, R.P. A Friedel–Crafts alkylation mechanism using an aminoindanol-derived thiourea catalyst. Org. Biomol. Chem. 2014, 12, 4503–4510. [Google Scholar] [CrossRef] [PubMed]
- Turks, M.; Rolava, E.; Stepanovs, D.; Mishnev, A.; Markovi, D. Novel 3-C-aminomethyl-hexofuranose-derived thioureas and their testing in asymmetric catalysis. Tetrahedron Asymmetry 2015, 26, 952–960. [Google Scholar] [CrossRef]
- McGuirk, C.M.; Mendez-Arroyo, J.; Lifschitz, A.M.; Mirkin, C.A. Allosteric Regulation of Supramolecular Oligomerization and Catalytic Activity via Coordination-Based Control of Competitive Hydrogen-Bonding Events. J. Am. Chem. Soc. 2014, 136, 16594–16601. [Google Scholar] [CrossRef] [PubMed]
- Schafer, A.G.; Wieting, J.M.; Mattson, A.E. Silanediols: A new class of hydrogen bond donor catalysts. Org. Lett. 2011, 13, 5228–5231. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Wilson, S.O.; Franz, A.K. First Catalytic Asymmetric [3+2] allylsilane annulation reaction Cooperative Hydrogen-Bonding Effects in Silanediol Catalysis. Org. Lett. 2012, 14, 186–189. [Google Scholar] [CrossRef] [PubMed]
- An, L.-T.; Zou, J.-P.; Zhang, L.-L.; Zhang, Y. Sulfamic acid-catalyzed Michael addition of indoles and pyrrole to electron-deficient nitroolefins under solvent-free condition. Tetrahedron Lett. 2007, 48, 4297–4300. [Google Scholar] [CrossRef]
- Moriyama, K.; Sugiue, T.; Saito, Y.; Katsuta, S.; Togo, H. 2,6-Bis(amido)benzoic Acid with Internal Hydrogen Bond as Brønsted Acid Catalyst for Friedel–Crafts Reaction of Indoles. Adv. Synth. Catal. 2015, 357, 2143–2149. [Google Scholar] [CrossRef]
- Bandini, M.; Garelli, A.; Rovinetti, M.; Tommasi, S.; Umani-Ronchi, A. Catalytic enantioselective addition of indoles to arylnitroalkenes: An effective route to enantiomerically enriched tryptamine precursors. Chirality 2005, 17, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Bisai, A.; Singh, V.K. Enantioselective Friedel–Crafts alkylation of indoles with nitroalkenes catalyzed by a bis(oxazoline)–Cu(II) complex. Tetrahedron Lett. 2007, 48, 1127–1129. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, S.; Oh, K. Orthogonal enantioselectivity approaches using homogeneous and heterogeneous catalyst systems: Friedel–Crafts alkylation of indole. Angew. Chem. Int. Ed. 2010, 49, 4476–4478. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Yokoyama, N. Tandem catalytic asymmetric Friedel–Crafts/Henry reaction: Control of three contiguous acyclic stereocenters. Angew. Chem. Int. Ed. 2008, 47, 4989–4992. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Yokoyama, N.; Yanagisawa, A. A library of chiral imidazoline–aminophenol ligands: Discovery of an efficient reaction sphere. Chem. Eur. J. 2008, 14, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, N.; Arai, T. Asymmetric Friedel–Crafts reaction of N-heterocycles and nitroalkenes catalyzed by imidazoline–aminophenol–Cu complex. Chem. Commun. 2009, 22, 3285–3257. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhu, S.; Yang, Y.; Zhou, Q.; August, R.V. Asymmetric Friedel−Crafts Alkylations of Indoles with Nitroalkenes Catalyzed by Zn(II)−Bisoxazoline Complexes. J. Org. Chem. 2006, 71, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Lai, G.; Xiong, S.; Wang, S.; Wang, Z. Monodentate N-Ligand-Directed Bifunctional Transition-Metal Catalysis: Highly Enantioselective Friedel–Crafts Alkylation of Indoles with Nitroalkenes. Chem. Eur. J. 2010, 16, 6438–6441. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.-L.; Lei, Z.-Y.; Shi, M. BINAM and H8-BINAM-based chiral imines and Zn(OTf)2-catalyzed enantioselective Friedel–Crafts alkylation of indoles with nitroalkenes. Tetrahedron Asymmetry 2008, 19, 1339–1346. [Google Scholar] [CrossRef]
- Lin, S.; You, T. Synthesis of 9,9′-biphenanthryl-10, 10′-bis (oxazoline)s and their preliminary evaluations in the Friedel–Crafts alkylations of indoles with nitroalkenes. Tetrahedron 2009, 65, 1010–1016. [Google Scholar] [CrossRef]
- McKeon, S.C.; Müller-Bunz, H.; Guiry, P.J. New Thiazoline–Oxazoline Ligands and Their Application in the Asymmetric Friedel–Crafts Reaction. Eur. J. Org. Chem. 2009, 2009, 4833–4841. [Google Scholar] [CrossRef]
- Jalal, S.; Sarkar, S.; Bera, K.; Maiti, S.; Jana, U. Synthesis of Nitroalkenes Involving a Cooperative Catalytic Action of Iron(III) and Piperidine: A One-Pot Synthetic Strategy to 3-Alkylindoles, 2H-Chromenes and N-Arylpyrrole. Eur. J. Org. Chem. 2013, 2013, 4823–4828. [Google Scholar] [CrossRef]
- Kitanosono, T.; Miyo, M.; Kobayashi, S. The combined use of cationic palladium(II) with a surfactant for the C–H functionalization of indoles and pyrroles in water. Tetrahedron 2015, 71, 7739–7744. [Google Scholar] [CrossRef]
- Zhan, Z.-P.; Yang, R.-F.; Lang, K. Samarium triiodide-catalyzed conjugate addition of indoles with electron-deficient olefins. Tetrahedron Lett. 2005, 46, 3859–3862. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Nowrouzi, F. The facile and efficient Michael addition of indoles and pyrrole to α,β-unsaturated electron-deficient compounds catalyzed by aluminium dodecyl sulfate trihydrate [Al(DS)3]·3H2O in water. Chem. Commun. 2005, 6, 789–791. [Google Scholar] [CrossRef] [PubMed]
- More, G.V.; Bhanage, B.M. Synthesis of a chiral fluorescence active probe and its application as an efficient catalyst in the asymmetric Friedel–Crafts alkylation of indole derivatives with nitroalkenes. Catal. Sci. Technol. 2015, 5, 1514–1520. [Google Scholar] [CrossRef]
- Guo, F.; Chang, D.; Lai, G.; Zhu, T.; Xiong, S.; Wang, S.; Wang, Z. Enantioselective and Regioselective Friedel–Crafts Alkylation of Pyrroles with Nitroalkenes Catalyzed by a Tridentate Schiff Base–Copper Complex. Chem. Eur. J. 2011, 17, 11127–11130. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Lin, L.; Liu, X.; Feng, X. Zinc Catalysis: Applications in Organic Synthesis; Enthaler, S., Wu, X.-F., Eds.; WILEY-VCH Verlag: Weinheim, Germany, 2015. [Google Scholar]
- Carmona, D.; Lamata, M.P.; Sánchez, A.; Viguri, F.; Oro, L.A. Enantioselective Friedel–Crafts alkylations catalysed by well-defined iridium and rhodium half-sandwich complexes. Tetrahedron Asymmetry 2011, 22, 893–906. [Google Scholar] [CrossRef] [Green Version]
- Méndez, I.; Rodríguez, R.; Polo, V.; Passarelli, V.; Lahoz, F.J.; García-Orduña, P.; Carmona, D. Temperature Dual Enantioselective Control in a Rhodium-Catalyzed Michael-Type Friedel–Crafts Reaction: A Mechanistic Explanation. Chem. Eur. J. 2016, 22, 11064–11083. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-W.; Liu, T.; Hu, Y.-Z.; Liu, X.-Y.; Che, C.-M. Urea postmodified in a metal–organic framework as a catalytically active hydrogen-bond-donating heterogeneous catalyst. Chem. Commun. 2013, 49, 7681–7683. [Google Scholar] [CrossRef] [PubMed]
- McGuirk, C.M.; Katz, M.J.; Stern, C.L.; Sarjeant, A.A.; Hupp, J.T.; Farha, O.K.; Mirkin, C.A. Turning on catalysis: Incorporation of a hydrogen-bond-donating squaramide moiety into a Zr metal-organic framework. J. Am. Chem. Soc. 2015, 137, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, S.; Jahanshahi, R. Nano n-propylsulfonated γ-Fe2O3 (NPS-γ-Fe2O3) as a magnetically recyclable heterogeneous catalyst for the efficient synthesis of 2-indolyl-1-nitroalkanes and bis(indolyl) methanes. New J. Chem. 2013, 37, 1009–1015. [Google Scholar] [CrossRef]
- Jeganathan, M.; Kanagaraj, K.; Dhakshinamoorthy, A.; Pitchumani, K. Michael addition of indoles to β-nitrostyrenes catalyzed by HY zeolite under solvent-free conditions. Tetrahedron Lett. 2014, 55, 2061–2064. [Google Scholar] [CrossRef]
- Lu, S.-F.; Du, D.-M.; Xu, J. Enantioselective Friedel−Crafts Alkylation of Indoles with Nitroalkenes Catalyzed by Bifunctional Tridentate Bis(oxazoline)−Zn(II) Complex. Org. Lett. 2006, 8, 2115–2118. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, D.-M. Development of Diphenylamine-Linked Bis(imidazoline) Ligands and Their Application in Asymmetric Friedel–Crafts Alkylation of Indole Derivatives with Nitroalkenes. Adv. Synth. Catal. 2010, 352, 1113–1118. [Google Scholar] [CrossRef]
- Liu, H.; Xu, J.; Du, D.M. Asymmetric Friedel-Crafts alkylation of methoxyfuran with nitroalkenes catalyzed by diphenylamine-tethered bis(oxazoline)-Zn(II) complexes. Org. Lett. 2007, 9, 4725–4728. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, S.F.; Xu, J.; Du, D.M. Asymmetric Friedel–Crafts Alkylation of Electron-Rich N-Heterocycles with Nitroalkenes Catalyzed by Diphenylamine-Tethered Bis(oxazoline) and Bis(thiazoline) ZnII Complexes. Chem. Asian J. 2008, 3, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, D.-M.M. Immobilization of Diphenylamine-Linked Bis(oxazoline) Ligands and Their Application in the Asymmetric Friedel–Crafts Alkylation of Indole Derivatives with Nitroalkenes. Eur. J. Org. Chem. 2010, 2010, 2121–2131. [Google Scholar] [CrossRef]
- Handa, S.; Gnanadesikan, V.; Matsunaga, S.; Shibasaki, M. syn-Selective Catalytic Asymmetric Nitro-Mannich Reactions Using a Heterobimetallic Cu−Sm−Schiff Base Complex. J. Am. Chem. Soc. 2007, 129, 4900–4901. [Google Scholar] [CrossRef] [PubMed]
- Maayan, G.; Christou, G. ‘Old’ Clusters with New Function: Oxidation Catalysis by High Oxidation State Manganese and Cerium/Manganese Clusters Using O2 Gas. Inorg. Chem. 2011, 50, 7015–7021. [Google Scholar] [CrossRef] [PubMed]
- Evangelisti, F.; More, R.; Hodel, F.; Luber, S.; Patzke, G.R. 3d–4f {CoII3Ln(OR)4} Cubanes as Bio-Inspired Water Oxidation Catalysts. J. Am. Chem. Soc. 2015, 137, 11076–11084. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Gallop, C.W.D.; Abdul-Sada, A.; Vargas, A.; Navarro, O.; Kostakis, G.E. Heteronuclear 3d/DyIII coordination clusters as catalysts in a domino reaction. Chem. Eur. J. 2015, 21, 6358–6361. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Kumar, P.; Mattock, J.D.; Abdul-Sada, A.; Pitak, M.B.; Coles, S.J.; Navarro, O.; Vargas, A.; Kostakis, G.E. Efficient NiII2LnIII2 Electrocyclization Catalysts for the Synthesis of trans-4,5-Diaminocyclopent-2-enones from 2-Furaldehyde and Primary or Secondary Amines. Inorg. Chem. 2016, 55, 6988–6994. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Kumar, P.; Akien, G.; Chilton, N.F.; Abdul-Sada, A.; Tizzard, G.J.; Coles, S.; Kostakis, G.E. Tetranuclear Zn/4f coordination clusters as highly efficient catalysts for Friedel–Crafts alkylation. Chem. Commun. 2016, 52, 7866–7869. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Dokorou, V.N.; Spencer, J.; Abdul-Sada, A.; Vargas, A.; Kostakis, G.E. Isoskeletal Schiff base polynuclear coordination clusters: Synthetic and theoretical aspects. CrystEngComm 2016, 18, 704–713. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Acta Crystallogr. Crystal structure refinement with SHELXL. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Van De Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]



| Entry | Catalyst | Yield (%) b |
|---|---|---|
| 1 | none | 0 |
| 2 | Dy (OTf)3 | 8 |
| 3 | Zn (OTf)2 | 20 |
| 4 | 1Dy | 99 |
| 5 | 1Y | 94 |
| 6 | 1Eu | 55 |
| 7 | 1Gd | 30 |
| 8 | 1Nd | 12 |
| 9 | 1Tb | 24 |
| 10 | 1Dy (Toluene) | 30 |
| 11 | 1Dy (Water) | 0 |
| 12 | 1Dy (THF) | 0 |
| 13 | 1Dy (Acetonitrile) | 0 |
| 14 | 1Dy (DMF) | 5 |
| 15 | 1Dy (−30 °C) | 0 |
| 16 | 1Dy (0 °C) | 5 |
| 17 | 1Dy (60 °C) | 30 |
| 18 | 1Dy (0.5%) | 26 |
| 19 | 1Dy (2.5%) | 62 |
| 20 | 1Dy (5.0%) | 17 |
| Entry | R1 | R2 | Yield (%) b |
|---|---|---|---|
| 1 | H | Ph | 99 (4a) |
| 2 | H | 4-Me-Ph | 98 (4b) |
| 3 | H | 4-MeO-Ph | 96 (4c) |
| 4 | H | 4-F-Ph | 92 (4d) |
| 5 | H | 4-Br-Ph | 94 (4e) |
| 6 | H | 2-furan | 99 (4f) |
| 7 | OMe | Ph | 97 (4g) |
| 8 | Br | Ph | 95 (4h) |
| 9 | I | Ph | 94 (4i) |
| 10 | NO2 | Ph | 76 (4j) |
| 11 | OCH2C6H5 | Ph | 96 (4k) |
| 12 | OMe | 2-furan | 98 (4l) |
| 13 | Br | 2-furan | 95 (4m) |
| 14 | OCH2C6H5 | 2-furan | 96 (4n) |
| 15 | I | 2-furan | 95 (4o) |
| Entry | R1 | R3 | R2 | Yield (%) b |
|---|---|---|---|---|
| 1 | CH3 | H | Ph | 99 (6a) |
| 2 | CH3 | H | 4-MeO-Ph | 99 (6b) |
| 3 | CH3 | H | 4-F-Ph | 93 (6c) |
| 4 | CH3 | H | 4-Br-Ph | 95 (6d) |
| 5 | CH3 | H | 2-furanyl | 99 (6e) |
| 6 | H | CH3 | Ph | 99 (6f) |
| 7 | H | CH3 | 4-F-Ph | 94 (6g) |
| 8 | H | CH3 | 4-Br-Ph | 96 (6h) |
| 9 | H | CH3 | 2-furanyl | 99 (6i) |
| 10 | H | CH3 | 4-Me-Ph | 98 (6j) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, P.; Lymperopoulou, S.; Griffiths, K.; Sampani, S.I.; Kostakis, G.E. Highly Efficient Tetranuclear ZnII2LnIII2 Catalysts for the Friedel–Crafts Alkylation of Indoles and Nitrostyrenes. Catalysts 2016, 6, 140. https://doi.org/10.3390/catal6090140
Kumar P, Lymperopoulou S, Griffiths K, Sampani SI, Kostakis GE. Highly Efficient Tetranuclear ZnII2LnIII2 Catalysts for the Friedel–Crafts Alkylation of Indoles and Nitrostyrenes. Catalysts. 2016; 6(9):140. https://doi.org/10.3390/catal6090140
Chicago/Turabian StyleKumar, Prashant, Smaragda Lymperopoulou, Kieran Griffiths, Stavroula I. Sampani, and George E. Kostakis. 2016. "Highly Efficient Tetranuclear ZnII2LnIII2 Catalysts for the Friedel–Crafts Alkylation of Indoles and Nitrostyrenes" Catalysts 6, no. 9: 140. https://doi.org/10.3390/catal6090140
APA StyleKumar, P., Lymperopoulou, S., Griffiths, K., Sampani, S. I., & Kostakis, G. E. (2016). Highly Efficient Tetranuclear ZnII2LnIII2 Catalysts for the Friedel–Crafts Alkylation of Indoles and Nitrostyrenes. Catalysts, 6(9), 140. https://doi.org/10.3390/catal6090140