Synthesis of Stereodiblock Polybutadiene Using Cp*Nd(BH4)2(thf)2 as a Catalyst
Abstract
:1. Introduction
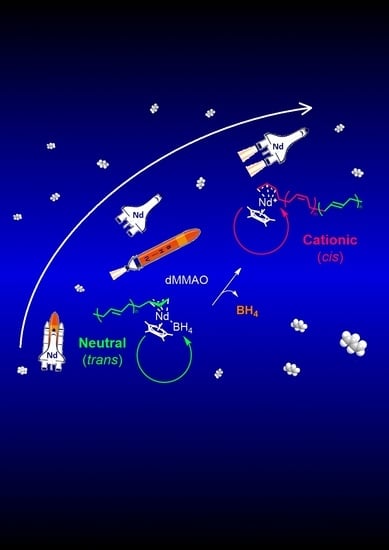
2. Results and Discussion
3. Experimental Section
3.1. General
3.2. Polymerization Procedure
3.2.1. Butadiene Polymerization Using the Cp*Nd(BH4)2(thf)2–Bu2Mg/dMMAO System
3.2.2. Synthesis of Stereoblock Polybutadiene Using Cp*Nd(BH4)2(thf)2 as a Catalyst
3.2.3. Determination of Stereoregularity of Polybutadiene
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brandt, H.D.; Nentwig, W.; Rooney, N.; LaFlair, R.T.; Wolf, U.U.; Duffy, J.; Puskas, J.E.; Kaszas, G.; Drewitt, M.; Glander, S. Rubber, 5. Solution Rubbers. In Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; Elvers, B., Ed.; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Hadjiandreou, P.; Julemont, M.; Teyssie, P. Butadiene 1,4-Polymerization Initiated by Bis[(η3-allyl)(trifluoroacetato)nickel]: A Perfectly “Living” Coordination System. Macromolecules 1984, 17, 2455–2456. [Google Scholar] [CrossRef]
- Cai, Z.; Shinzawa, M.; Nakayama, Y.; Shiono, T. Synthesis of Regioblock Polybutadiene with CoCl2-Based Catalyst via Reversible Coordination of Lewis Base. Macromolecules 2009, 42, 7642–7643. [Google Scholar] [CrossRef]
- Tanaka, R.; Kasai, Y.; Shinzawa, M.; Cai, Z.; Nakayama, Y.; Shiono, T. Synthesis of multiblock copolymer of poly(cis-1,4-butadiene) and poly(3-buten-1-ol). Macromol. Chem. Phys. 2014, 215, 888–892. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, F.; Bi, J.; Zhang, H.; Zhang, C.; Hu, Y.; Bai, C.; Zhang, X. Synthesis and Characterization of Soft–Hard Stereoblock Polybutadiene with Fe(2-EHA)3/Al(i-Bu)3/DEP Catalyst System. J. Polym. Chem. Part A Polym. Chem. 2015, 53, 1182–1188. [Google Scholar] [CrossRef]
- Phuphuak, Y.; Bonnet, F.; Stoclet, G.; Briad, M.; Zinck, P. Isoprene chain shuttling polymerisation between cis and trans regulating catalysts: Straightforward access to a new material. Chem. Commun. 2017, 53, 5330–5333. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Yuuya, K.; Sato, H.; Eberhardt, P.; Nakayama, Y.; Shiono, T. Synthesis of stereodiblock polyisoprene consisting of cis-1,4 and trans-1,4 sequences by using a neodymium catalyst: Change of the stereospecificity triggered by an aluminum compound. Polym. Chem. 2016, 7, 1239–1243. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, D.; Wang, B.; Liu, B.; Yang, Y. Polymerization of 1,3-Conjugated Dienes with Rare-Earth Metal Precursors. Mol. Catal. Rare-Earth Elem. 2010, 137, 49–108. [Google Scholar] [CrossRef]
- Maiwald, S.; Weißenborn, H.; Sommer, C.; Müller, G.; Taube, R. Komplexkatalyse LIX. Die Katalyse der 1,4-trans-Polymerisation des Butadiens mit Tris(allyl)neodym(III) Nd(η3-C3H5)3 als Einkomponentenkatalysator—Kinetik und Reaktionsmechanismus. J. Organomet. Chem. 2001, 640, 1–9. [Google Scholar] [CrossRef]
- Bonnet, F.; Visseaux, M.; Pereira, A.; Barbier-Baudry, D. Highly trans-Stereospecific Isoprene Polymerization by Neodymium Borohydrido Catalysts. Macromolecules 2005, 38, 3162–3169. [Google Scholar] [CrossRef] [Green Version]
- Ventura, A.; Chenal, T.; Bria, M.; Bonnet, F.; Zinck, P.; Ngono-Ravache, Y.; Balanzat, E.; Visseaux, M. Trans-stereospecific polymerization of butadiene and random copolymerization with styrene using borohydrido neodymium/magnesium dialkyl catalysts. Eur. Polym. J. 2013, 49, 4130–4140. [Google Scholar] [CrossRef] [Green Version]
- Fadlallah, S.; Terrier, M.; Jones, C.; Roussel, P.; Bonnet, F.; Visseaux, M. Mixed Allyl−Borohydride Lanthanide Complexes: Synthesis of Ln(BH4)2(C3H5)(THF)3 (Ln = Nd, Sm), Characterization, and Reactivity toward Polymerization. Organometallics 2016, 35, 456–461. [Google Scholar] [CrossRef]
- Maiwald, S.; Sommer, C.; Müller, G.; Taube, R. On the 1,4-cis-Polymerization of Butadiene with the Highly Active Catalyst Systems Nd(C3H5)2Cl·1.5 THF/Hexaisobutylaluminoxane (HIBAO), Nd(C3H5)Cl2·2 THF/HIBAO and Nd(C3H5)Cl2·2 THF/Methylaluminoxane (MAO)—Degree of Polymerization, Polydispersity, Kinetics and Catalyst Formation. Macromol. Chem. Phys. 2001, 202, 1446–1456. [Google Scholar]
- Ajellal, N.; Furlan, L.; Thomas, C.M.; Casagrande, O.L., Jr.; Carpentier, J.F. Mixed Aluminum-Magnesium-Rare Earth Allyl Catalysts for Controlled Isoprene Polymerization: Modulation of Stereocontrol. Macromol. Rapid Commun. 2006, 27, 338–343. [Google Scholar] [CrossRef]
- Conti, F.; Segre, A.; Pini, P.; Porri, L. Nuclear magnetic resonance studies of polydienes: 1. 13C n.m.r, of 1,4-polybutadiene obtained by π-allyl nickel trifluoroacetate catalysts. Polymer 1974, 15, 5–8. [Google Scholar] [CrossRef]
- Visseaux, M.; Mainil, M.; Terrier, M.; Mortreux, A.; Roussel, P.; Mathivet, T.; Destarac, M. Cationic borohydrido–neodymium complex: Synthesis, characterization and its application as an efficient pre-catalyst for isoprene polymerization. Dalton Trans. 2008, 4558–4561. [Google Scholar] [CrossRef]
- Valente, A.; Zinck, P.; Mortreux, A.; Visseaux, M. Borohydrido Rare Earth Based Coordinative Chain Transfer Copolymerization: A Convenient Tool for Tuning the Microstructure of Isoprene/Styrene Copolymers. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1615–1620. [Google Scholar] [CrossRef]
- Tanaka, R.; Kawahara, T.; Shinto, Y.; Nakayama, Y.; Shiono, T. An alternative method for the preparation of trialkylaluminum-depleted modified-methylaluminoxane (dMMAO). Macromolecules 2017, 50, 5989–5993. [Google Scholar] [CrossRef]
- Cendrowski-Guillaume, S.M.; Le Gland, G.; Nierlich, M.; Ephritikhine, M. Lanthanide Borohydrides as Precursors to Organometallic Compounds. Mono(cyclooctatetraenyl) Neodymium Complexes. Organometallics 2000, 19, 5654–5660. [Google Scholar] [CrossRef]









| Run | Nd (μmol) | Al/Mg (mol/mol) | Time (h) | Yield (%) | Mna (×104) | Mw/Mna | trans:cis:vinyl b (mol %) |
|---|---|---|---|---|---|---|---|
| 1 c | 25 | 0 | 1 | 20 | 0.8 | 1.3 | 93:3:4 |
| 2 | 25 | 25 | 1 | 85 | 8.2 | 1.5 | 34:61:5 |
| 3 d | 25 | 25 | 0.5 | 70 | 7.9 | 1.7 | 15:81:4 |
| 4 | 25 | 50 | 1 | 84 | 10.2 | 1.7 | 13:84:3 |
| 5 | 25 | 100 | 12 | 28 | 2.7 | 1.7 | 6:92:2 |
| 6 e | 50 | 25 | 1.5 | 93 | 5.2 | 2.5 | 59:38:3 |

| Run | Bd Total (mmol) | Bd/Nd (mol/mol) | Yield (%) | Mna (×104) | PDIa | Trans:Cis:Vinyl b (mol %) | N c |
|---|---|---|---|---|---|---|---|
| 7 | 8 | 320 | 98 | 4.9 | 1.9 | 14:83:3 | 0.35 |
| 8 | 16 | 640 | 84 | 10.2 | 1.7 | 13:84:3 | 0.28 |
| 9 | 32 | 1280 | 73 | 16.0 | 1.7 | 20:76:4 | 0.32 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, R.; Shinto, Y.; Nakayama, Y.; Shiono, T. Synthesis of Stereodiblock Polybutadiene Using Cp*Nd(BH4)2(thf)2 as a Catalyst. Catalysts 2017, 7, 284. https://doi.org/10.3390/catal7100284
Tanaka R, Shinto Y, Nakayama Y, Shiono T. Synthesis of Stereodiblock Polybutadiene Using Cp*Nd(BH4)2(thf)2 as a Catalyst. Catalysts. 2017; 7(10):284. https://doi.org/10.3390/catal7100284
Chicago/Turabian StyleTanaka, Ryo, Yuto Shinto, Yuushou Nakayama, and Takeshi Shiono. 2017. "Synthesis of Stereodiblock Polybutadiene Using Cp*Nd(BH4)2(thf)2 as a Catalyst" Catalysts 7, no. 10: 284. https://doi.org/10.3390/catal7100284
APA StyleTanaka, R., Shinto, Y., Nakayama, Y., & Shiono, T. (2017). Synthesis of Stereodiblock Polybutadiene Using Cp*Nd(BH4)2(thf)2 as a Catalyst. Catalysts, 7(10), 284. https://doi.org/10.3390/catal7100284