Ene-yne Cross-Metathesis for the Preparation of 2,3-Diaryl-1,3-dienes
Abstract
:1. Introduction
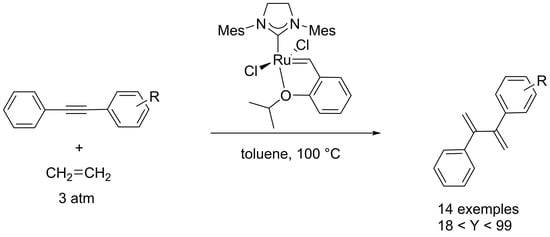
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References and Notes
- Hayashi, S.; Kasuya, M.; Machida, J.; Koizumi, T. From propargylic biscarbonate to diaryl[n]dendralenes. Tetrahedron Lett. 2017, 58, 2429–2432. [Google Scholar] [CrossRef]
- Hopf, H.; Sherburn, M.S. Dendralenes branch out: Cross-conjugated oligoenes allow for rapid generation of molecular complexity. Angew. Chem. Int. Ed. 2012, 51, 2298–2338. [Google Scholar] [CrossRef] [PubMed]
- Sherburn, M.S. Preparation and synthetic value of n-bond rich branched hydrocarbons. Acc. Chem. Res. 2015, 48, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Kotha, S.; Meshram, M.; Tiwari, A. Advanced approach to polycyclics by a synergetic combination of enyne metathesis and Diels-Alder reaction. Chem. Soc. Rev. 2009, 38, 2065–2092. [Google Scholar] [CrossRef] [PubMed]
- Omura, S.; Fukuyama, T.; Horiguchi, J.; Murakami, Y.; Ryu, I. Ruthenium hydride-catalyzed addition of aldehydes to dienes leading to β,γ-unsaturated ketones. J. Am. Chem. Soc. 2008, 130, 14094–14095. [Google Scholar] [CrossRef] [PubMed]
- Balla, A.; Al-Hashimi, M.; Hlil, A.; Bazzi, H.; Tuba, R. Ruthenium-catalyzed metathesis of conjugated polyenes. ChemCatChem 2016, 8, 2865–2875. [Google Scholar] [CrossRef]
- Mori, Y.; Mori, T.; Onodera, G.; Kimura, M. Nickel-catalyzed multicomponent coupling of alkyne, buta-1,3-diene, and dimethylzinc under carbon dioxide. Synthesis 2014, 46, 2287–2292. [Google Scholar] [CrossRef]
- Takimoto, M.; Kajima, Y.; Sato, Y.; Mori, M. Nickel-catalyzed enantioselective three-component coupling of bis-1,3-dienes, aldehydes, and dimethylzinc. J. Org. Chem. 2005, 70, 8605–8608. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.D.; Denton, J.R.; Lian, Y.; Davies, H.M.L.; Williams, C.M. Asymmetric [4 + 3] cycloaddition between vinylcarbenoids and dienes: Application to the total synthesis of the natural product (6)-5-epi-vibsanin E. J. Am. Chem. Soc. 2009, 131, 8329–8332. [Google Scholar] [CrossRef] [PubMed]
- Torrente-Murciano, L.; Lapkin, A.; Nielsen, D.J.; Fallis, I.; Cavell, K.J. Telomerisation of long-chain dienes with alcohols using Pd(IMes)(dvds) catalyst. Green Chem. 2010, 12, 866–869. [Google Scholar] [CrossRef]
- Behr, A.; Becker, M.; Beckmann, T.; Johnen, L.; Leschinski, J.; Reyer, S. Telomerization: Advances and applications of a versatile reaction. Angew. Chem. Int. Ed. 2009, 48, 3598–3614. [Google Scholar] [CrossRef] [PubMed]
- Neubert, P.; Meier, I.; Gaide, T.; Kuhlmann, R.; Behr, A. First telomerisation of piperylene with morpholine using palladium-carbene catalysts. Catal. Commun. 2016, 77, 70–74. [Google Scholar] [CrossRef]
- Neubert, P.; Meier, I.; Gaide, T.; Behr, A. Additive-free palladium-catalysed hydroamination of piperylene with morpholine. Synthesis 2016, 48, 2287–2293. [Google Scholar]
- Behr, A.; Neubert, P. Piperylene—A versatile basic chemical in catalysis. ChemCatChem 2014, 6, 412–428. [Google Scholar] [CrossRef]
- Li, H.; Fang, X.; Jackstell, R.; Neumann, H.; Beller, M. Palladium-catalysed hydroamidocarbonylation of 1,3-dienes. Chem. Commun. 2016, 52, 7142–7145. [Google Scholar] [CrossRef] [PubMed]
- Eom, D.; Park, S.; Park, Y.; Ryu, T.; Lee, P.H. Synthesis of indenes via Bronsted acid-catalyzed cyclization of diaryl-and alkyl aryl-1,3-dienes. Org. Lett. 2012, 14, 5392–5395. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.R.; Griffiths, J.R.; Diver, S.T. Ruthenium hydride-promoted dienyl isomerization: Access to highly substituted 1,3-dienes. J. Am. Chem. Soc. 2013, 135, 3327–3330. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Sigman, M.S. Palladium-catalyzed hydroarylation of 1,3-dienes with boronic esters via reductive formation of π-allyl palladium intermediates under oxidative conditions. J. Am. Chem. Soc. 2010, 132, 10209–10211. [Google Scholar] [CrossRef] [PubMed]
- Raya, B.; Jing, S.; Balasanthiran, V.; RajanBabu, T.V. Control of selectivity through synergy between catalysts, silanes, and reaction conditions in cobalt-catalyzed hydrosilylation of dienes and terminal alkenes. ACS Catal. 2017, 7, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; RajanBabu, T.V. Asymmetric hydrovinylation of unactivated linear 1,3-dienes. J. Am. Chem. Soc. 2010, 132, 3295–3297. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Fujiwara, D.; Endo, K. Rh-catalyzed intermolecular and enantioselective [4 + 2] cycloaddition of 1,3-dienes with dimethyl acetylenedicarboxylate. Org. Biomol. Chem. 2008, 6, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Kotha, S.; Seema, V. Diversity-oriented synthesis of biaryl derivatives using cross-enyne metathesis, Diels Alder reaction, and Suzuki-Miyaura cross-coupling as key steps. Synlett 2011, 2329–2334. [Google Scholar] [CrossRef]
- Lewis, R.B.; de Alaniz, J.R. Nitrosocarbonyl hetero-Diels-Alder cycloaddition with 2-substituted 1,3-butadienes. Tetrahedron 2017, 73, 4045–4051. [Google Scholar] [CrossRef]
- Srour, H.; Abidi, K.; Sahli, Z.; Sundararaju, B.; Hamdi, N.; Achard, M.; Bruneau, C. Dendralenes preparation via ene-yne cross-metathesis from in situ generated 1,3-enynes. ChemCatChem 2011, 3, 1876–1879. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Herkommer, D.; Krische, M.J. Enantioselective formation of all-carbon quaternary centers via C-H functionalization of methanol: Iridium-catalyzed diene hydrohydroxymethylation. J. Am. Chem. Soc. 2016, 138, 14210–14213. [Google Scholar] [CrossRef] [PubMed]
- Smejkal, T.; Han, H.; Breit, B.; Krische, M.J. All-carbon quaternary centers via ruthenium-catalyzed hydromethylation of 2-substituted butadienes mediated by formaldehyde: Beyond hydroformylation. J. Am. Chem. Soc. 2009, 131, 10366–10367. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, J.; Machida, H.; Saito, R.; Akimoto, K.; Hoshino, M. Efficient preparation of polysubstituted 1,3-dienes. Chem. Lett. 1985, 14, 1173–1176. [Google Scholar] [CrossRef]
- Hu, Y.; Li, N.; Li, G.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Solid acid-catalyzed dehydration of pinacol derivatives in ionic liquid: Simple and efficient access to branched 1,3-dienes. ACS Catal. 2017, 7, 2576–2582. [Google Scholar] [CrossRef]
- Böhmer, J.; Grigg, R. Pd(0)-catalysed formation of diarylated dienes from propargyl carbonates and organoboron and organotin(IV) reagents. Tetrahedron 1999, 55, 13463–13470. [Google Scholar] [CrossRef]
- Green, N.J.; Willis, A.C.; Sherburn, M.S. Direct cross-coupling of propargylic diols. Angew. Chem. Int. Ed. 2016, 55, 9244–9248. [Google Scholar] [CrossRef] [PubMed]
- Ishino, Y.; Nishiguchi, I.; Takihira, F.; Hirashima, T. Novel synthesis of 2,3-diarylbuta-1,3-dienes from 1,4-dimethoxybutyne-2. Tetrahedron Lett. 1980, 21, 1527–1528. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Jia, X. Direct one-pot synthesis of 2,3-diarylbuta-1,3-dienes via self-coupling of acetophenones. Synlett 2008, 1529–1531. [Google Scholar] [CrossRef]
- Addie, M.S.; Taylor, R.J.K. 1,3-Dienes from ketones via the Shapiro reaction. ARKIVOC 2000, 2000, 660–666. [Google Scholar]
- Ojha, D.P.; Prabhu, K.R. Pd-catalyzed cross-coupling reactions of hydrazones: Regioselective synthesis of highly branched dienes. J. Org. Chem. 2013, 78, 12136–12143. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; He, L.; Li, X.; Chen, H.; Wu, W.; Fu, W. Facile synthesis of dibranched conjugated dienes via palladium-catalyzed oxidative coupling of N-tosylhydrazones. Chem. Commun. 2013, 49, 9218–9220. [Google Scholar] [CrossRef] [PubMed]
- Amaya, T.; Suzuki, R.; Hirao, T. Quinonediimines as redox-active organocatalysts for oxidative coupling of aryl- and alkenylmagnesium compounds under molecular oxygen. Chem. Commun. 2016, 52, 7790–7793. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Seomoon, D.; Lee, K. Palladium-catalyzed inter- and intramolecular coupling reactions of aryl and vinyl halides mediated by indium. Org. Lett. 2005, 7, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Celedon, C.A.; Rincon-Medina, J.A.; Mendoza-Rayo, D.; Chacon-Garcia, L. Oxidative homocoupling of arylboronic acids catalyzed by a 4-aminoantipyrine-Pd(II) complex. Appl. Organomet. Chem. 2015, 29, 439–442. [Google Scholar] [CrossRef]
- Ikeda, Z.; Oshima, K.; Matsuatma, S. Preparation and reaction of 2-aryl-3-silyl-1,3-butadiene. Org. Lett. 2005, 7, 4859–4861. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Kurahashi, T.; Shimono, K.; Tanaka, K.; Nagao, I.; Kiyomoto, S.-I.; Hiyama, T. Facile synthesis and palladium-catalyzed cross-coupling reactions of 2,3-bis(pinacolatoboryl)-1,3-butadiene. Chem. Asian J. 2007, 2, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Ansari, N.H.; Dacko, C.A.; Akhmedov, N.G.; Söderberg, B.C.G. Double Palladium Catalyzed Reductive Cyclizations. Synthesis of 2,2′-, 2,3′-, and 3,3′-Bi-1H-indoles, Indolo[3,2-b]indoles, and Indolo[2,3-b]indoles. J. Org. Chem. 2016, 81, 9337–9349. [Google Scholar] [CrossRef] [PubMed]
- Fischmeister, C.; Bruneau, C. Ene-yne cross-metathesis with ruthenium carbene catalysts. Beilstein J. Org. Chem. 2011, 7, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Mori, M. Enyne metathesis. Top. Organomet. Chem. 1998, 1, 133–154. [Google Scholar]
- Mori, M. Ruthenium-catalyzed ROM, RCM and CM of enyne. J. Mol. Catal. A: Chem. 2004, 213, 73–79. [Google Scholar] [CrossRef]
- Diver, S.T.; Griffiths, J.R. Ene-yne metathesis. In Olefin Metathesis: Theory and Practice; Grela, K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 153–185. ISBN 978-1-118-20794-9. [Google Scholar]
- Kinoshita, A.; Sakakiatma, N.; Mori, M. Novel 1,3-diene synthesis from alkyne and ethylene by ruthenium-catalyzed enyne metathesis. J. Am. Chem. Soc. 1997, 119, 12388–12389. [Google Scholar] [CrossRef]
- Galan, B.R.; Giessert, A.J.; Keister, J.B.; Diver, S.T. Studies on the mechanism of intermolecular enyne metathesis: Kinetic method and alkyne substituent effect. J. Am. Chem. Soc. 2005, 127, 5762–5763. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.R.; Keister, J.B.; Diver, S.T. From resting state to the steady state: Mechanistic studies of the ene-yne metathesis promoted by the Hoveyda complex. J. Am. Chem. Soc. 2016, 138, 5380–5391. [Google Scholar] [CrossRef] [PubMed]
- Smulik, J.A.; Diver, S.T. Expanded scope in ethylene-alkyne cross-metathesis: Coordinating heteroatom functionality at the propargylic position. Org. Lett. 2000, 2, 2271–2274. [Google Scholar] [CrossRef] [PubMed]
- Smulik, J.A.; Diver, S.T. Terminal-alkyne-ethylene cross-metathesis: Reaction of 1-substituted propargyl esters at elevated ethylene pressure. J. Org. Chem. 2000, 65, 1788–1792. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Sakakiatma, N.; Mori, M. Novel 1,3-diene synthesis from alkyne and ethylene by ruthenium-catalyzed enyne metathesis. Tetrahedron 1999, 55, 8155–8167. [Google Scholar] [CrossRef]
- Achard, M.; Derrien, N.; Demerseman, B.; Zhang, H.-J.; Bruneau, C. Ruthenium-catalyzed synthesis of functionalized 1,3-dienes. Org. Lett. 2009, 11, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Tonogaki, K.; Mori, M. An improved 1,3-diene synthesis from alkyne and ethylene using cross-enyne metathesis. Tetrahedron Lett. 2002, 43, 2235–2238. [Google Scholar] [CrossRef]
- Lauchli, R.; Shea, K.J. A synthesis of the Welwistatin core. Org. Lett. 2006, 8, 5287–5289. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, S.; Sariaslan, B.; Öztürk, B.Ö. A ruthenium-based catalytic system with switchable selectivity between cyclotrimerization and enyne metathesis/Diels-Alder reactions of terminal alkynes. Catal. Commun. 2013, 41, 12–16. [Google Scholar] [CrossRef]
- Sauvage, X.; Borguet, Y.; Noels, A.F.; Delaude, L.; Demonceau, A. Homobimetallic Ruthenium–N-Heterocyclic carbene complexes: Synthesis, characterization, and catalytic applications. Adv. Synth. Catal. 2007, 349, 255–265. [Google Scholar] [CrossRef]
- Hilt, G.; Roesner, S. Substrate-controlled regioselective cobalt(I)-catalysed 1,4-hydrovinylation reactions. Synthesis 2011, 4, 662–668. [Google Scholar] [CrossRef]
- Yi, C.S.; Lee, D.W.; Chen, Y. Hydrovinylation and [2 + 2] cycloaddition reactions of alkynes and alkenes catalyzed by a weel-defined cationic ruthenium-alkylidene complex. Organometallics 1999, 18, 2043–2045. [Google Scholar] [CrossRef]
- Miao, X.; Fischmeister, C.; Bruneau, C.; Dixneuf, P.H. Dimethyl carbonate: An eco-friendly solvent in ruthenium-catalyzed olefin metathesis transformations. ChemSusChem 2008, 1, 813–816. [Google Scholar] [CrossRef] [PubMed]
- The lower reactivity of first generation catalyst in ene-yne cross-metathesis with ethylene had previously been established with propargylic esters. See Ref. [45].




| Entry | Catalyst (mol%) | Ethylene Pressure (atm) | Solvent | T (°C) | t (h) | Conversion c (%) |
|---|---|---|---|---|---|---|
| 1 | I (2 mol%) | 1 | DMC 0.01 M | 25 | 4 | 0 |
| 2 | I (2 mol%) | 1 | DCM 0.06 M | 25 | 4 | 0 |
| 3 | I (2 mol%) | 1 | DMC 0.06 M | 25 | 4 | 5 |
| 4 | I (2 mol%) | 5 | DMC 0.06 M | 80 | 17 | 60 d |
| 5 | I (2 mol%) | 5 | toluene 0.06 M | 100 | 17 | 64 d |
| 6 | I (2 mol%) | 7 | DMC 0.06 M | 80 | 22 | 90 d |
| 7 | II (2 mol%) | 5 | DMC 0.06 M | 80 | 24 | 50 d |
| 8 | II (2 mol%) | 5 | DMC 0.1 M | 80 | 23 | 94 d |
| 9 | II (2 mol%) | 5 | DMC 0.3 M | 90 b | 17 | 92 d |
| 10 | II (2 mol%) | 3 | DMC 0.3 M | 90 b | 17 | 95 d |
| 11 | II (2 mol%) | 3 | toluene 0.3 M | 100 | 17 | 100 (90%) e |
| 12 | II (2 mol%) | 3 | toluene 0.3 M | 70 | 17 | 67 |
| 13 | II (2 mol%) | 3 | toluene 0.3 M | 120 | 8 | 90 |
| 14 | II (2 mol%) | 25 | toluene 0.3 M | 100 | 72 | 0 |
| 15 | II (1 mol%) | 3 | toluene 0.3 M | 100 | 17 | 80 |
| Entry | Substrate | t (h) | Conversion (%) b | Product | Yield (%) c | ||
|---|---|---|---|---|---|---|---|
| 1 |  | 1b | 24 | 100 |  | 2b | 91 |
| 2 |  | 1c | 17 | 100 |  | 2c | 98 |
| 3 |  | 1d | 17 | 100 |  | 2d | 99 |
| 4 |  | 1e | 17 | 100 |  | 2e | 91 |
| 5 |  | 1f | 17 | 100 |  | 2f | 99 |
| 6 |  | 1g | 24 | 100 |  | 2g | 97 |
| 7 |  | 1h | 24 | 92 |  | 2h | 92 |
| 8 |  | 1i | 17 | 100 |  | 2i | 90 |
| 9 |  | 1j | 22 | 100 |  | 2j | 92 |
| 10 |  | 1k | 17 | 100 |  | 2k | 91 |
| 11 |  | 1l | 27 | 100 |  | 2l | 60 |
| 12 |  | 1m | 48 | 40 |  | 2m | 18 |
| 13 |  | 1n | 24 | 0 |  | 2n | 0 |
| 14 |  | 1o | 24 | 0 |  | 2o | 0 |
| 15 |  | 1p | 24 | 100 |  | 2p | 97 |
| 16 |  | 1q | 17–48 | 10 |  | 2q | 0 |
| 17 |  | 1r | 24 | 0 |  | 2r | 0 |
| 18 |  | 1s | 17–48 | 0 |  | 2s | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abderrezak, M.K.; Kabouche, Z.; Bruneau, C.; Fischmeister, C. Ene-yne Cross-Metathesis for the Preparation of 2,3-Diaryl-1,3-dienes. Catalysts 2017, 7, 365. https://doi.org/10.3390/catal7120365
Abderrezak MK, Kabouche Z, Bruneau C, Fischmeister C. Ene-yne Cross-Metathesis for the Preparation of 2,3-Diaryl-1,3-dienes. Catalysts. 2017; 7(12):365. https://doi.org/10.3390/catal7120365
Chicago/Turabian StyleAbderrezak, Meriem K., Zahia Kabouche, Christian Bruneau, and Cédric Fischmeister. 2017. "Ene-yne Cross-Metathesis for the Preparation of 2,3-Diaryl-1,3-dienes" Catalysts 7, no. 12: 365. https://doi.org/10.3390/catal7120365
APA StyleAbderrezak, M. K., Kabouche, Z., Bruneau, C., & Fischmeister, C. (2017). Ene-yne Cross-Metathesis for the Preparation of 2,3-Diaryl-1,3-dienes. Catalysts, 7(12), 365. https://doi.org/10.3390/catal7120365