Engineering Pyrite-Type Bimetallic Ni-Doped CoS2 Nanoneedle Arrays over a Wide Compositional Range for Enhanced Oxygen and Hydrogen Electrocatalysis with Flexible Property
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Electrocatalytic Performance
2.3. Flexible Property
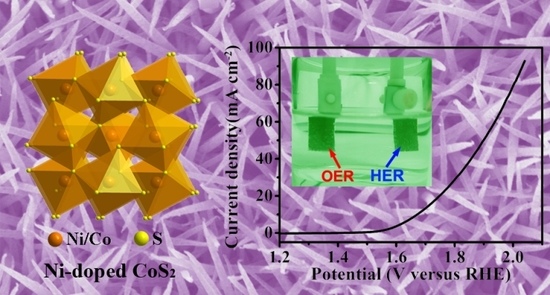
2.4. Overall Water-Splitting
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.2. Characterization
4.3. Electrochemical Tests
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nocera, D.G. The Artificial Leaf. Acc. Chem. Rev. 2012, 45, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Morales-Guio, C.G.; Stern, L.-A.; Hu, X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 2014, 43, 6555–6569. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar Energy Supply and Storage for the Legacy and Nonlegacy Worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Han, X.P.; Cheng, F.Y.; Zhang, T.R.; Yang, J.G.; Hu, Y.X.; Chen, J. Hydrogenated uniform Pt clusters supported on porous CaMnO3 as a bifunctional electrocatalyst for enhanced oxygen reduction and evolution. Adv. Mater. 2014, 26, 2047–2051. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, Q.; Asiri, A.M.; Sun, X. Self-Supported Nanoporous Cobalt Phosphide Nanowire Arrays: An Efficient 3D Hydrogen-Evolving Cathode over the Wide Range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, W.; Sun, A.; Qi, C.; Zhang, D.; Wu, Z.; Wang, D. Sulfur-Decorated Molybdenum Carbide Catalysts for Enhanced Hydrogen Evolution. ACS Catal. 2015, 5, 6956–6963. [Google Scholar] [CrossRef]
- Liu, Y.P.; Yu, G.T.; Li, G.D.; Sun, Y.H.; Asefa, T.; Chen, W.; Zou, X.X. Coupling Mo2C with Nitrogen-Rich Nanocarbon Leads to Efficient Hydrogen-Evolution Electrocatalytic Sites. Angew. Chem. Int. Ed. 2015, 54, 10752–10757. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Peng, Z.; Ye, R.; Zhou, H.; Guo, X. M3C (M: Fe, Co, Ni) Nanocrystals Encased in Graphene Nanoribbons: An Active and Stable Bifunctional Electrocatalyst for Oxygen Reduction and Hydrogen Evolution Reactions. ACS Nano 2015, 9, 7407–7418. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Tian, C.; Wang, L.; Wu, A.; Meng, M.; Zhao, L.; Fu, H. Phosphorus-Modified Tungsten Nitride/Reduced Graphene Oxide as a High-Performance, Non-Noble-Metal Electrocatalyst for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2015, 54, 6325–6329. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhao, Y.; Chen, G.; Shang, L.; Shi, R.; Kang, X.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Ni3FeN Nanoparticles Derived from Ultrathin NiFe-Layered Double Hydroxide Nanosheets: An Efficient Overall Water Splitting Electrocatalyst. Adv. Energy Mater. 2016, 6, 1502585. [Google Scholar] [CrossRef]
- Staszak-Jirkovsky, J.; Malliakas, C.D.; Lopes, P.P.; Danilovic, N.; Kota, S.S.; Chang, K.-C.; Genorio, B.; Strmcnik, D.; Stamenkovic, V.R.; Kanatzidis, M.G.; et al. Design of active and stable Co-Mo-Sx chalcogels as pH-universal catalysts for the hydrogen evolution reaction. Nat. Mater. 2016, 15, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.S.; Lukowski, M.A.; Ding, Q.; Kaiser, N.S.; Jin, S. Earth-abundant metal pyrites (FeS2, CoS2, NiS2, and their alloys) for highly efficient hydrogen evolution and polysulfide reduction electrocatalysis. J. Phys. Chem. C 2014, 118, 21347–21356. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S. Cobalt sulfide nanosheet/graphene/carbon nanotube nanocomposites as flexible electrodes for hydrogen evolution. Angew. Chem. Int. Ed. 2014, 53, 12594–12599. [Google Scholar]
- Wu, X.Y.; Han, X.P.; Ma, X.Y.; Zhang, W.; Deng, Y.D.; Zhong, C.; Hu, W.B. Morphology-Controllable Synthesis of Zn-Co-Mixed Sulfide Nanostructures on Carbon Fiber Paper Toward Efficient Rechargeable Zinc-Air Batteries and Water Electrolysis. ACS Appl. Mater. Interfaces 2017, 9, 12574–12583. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, H.; Lu, Z.; Cui, Y. CoSe2 Nanoparticles Grown on Carbon Fiber Paper: An Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2014, 136, 4897–4900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, B.; Wu, X.; Li, Z.; Lei, L.; Zhang, X. Polymorphic CoSe2 with Mixed Orthorhombic and Cubic Phases for Highly Efficient Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2015, 7, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Caban-Acevedo, M.; Stone, M.L.; Schmidt, J.R.; Thomas, J.G.; Ding, Q.; Chang, H.C.; Tsai, M.L.; He, J.H.; Jin, S. Efficient hydrogen evolution catalysis using ternary pyrite-type cobalt phosphosulphide. Nat. Mater. 2015, 14, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Chen, S.; Vasileff, A.; Qiao, S.Z. Anion and Cation Modulation in Metal Compounds for Bifunctional Overall Water Splitting. ACS Nano 2016, 10, 8738–8745. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Zhang, Y.J.; Hu, F.; Wang, Q.B. Urchin-like CoP nanocrystals as hydrogen evolution reaction and oxygen reduction reaction dual-electrocatalyst with superior stability. Nano Lett. 2015, 15, 7616–7620. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Y.; Li, G.-D.; Zou, X.; Lian, X.; Wang, D.; Sun, L.; Asefa, T.; Zou, X. Efficient electrocatalysis of overall water splitting by ultrasmall NixCo3−xS4 coupled Ni3S2 nanosheet arrays. Nano Energy 2017, 35, 161–170. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Gong, M.; Chou, H.-L.; Pan, C.-J.; Chen, H.-A.; Wu, Y.; Lin, M.-C.; Guan, M.; Yang, J.; Chen, C.-W.; et al. Highly Active and Stable Hybrid Catalyst of Cobalt-Doped FeS2 Nanosheets-Carbon Nanotubes for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2015, 137, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, J.; Yanzhang, R.; Du, M.; Wang, Q.; Gao, G.; Wu, J.; Wu, G.; Zhang, M.; Liu, B.; et al. When Cubic Cobalt Sulfide Meets Layered Molybdenum Disulfide: A Core-Shell System Toward Synergetic Electrocatalytic Water Splitting. Adv. Mater. 2015, 27, 4752–4759. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Peng, S.; Safanama, D.; Yu, H.; Li, L.; Yang, G.; Qin, X.; Srinivasan, M.; Adams, S.; Ramakrishna, S. Design of 3-Dimensional Hierarchical Architectures of Carbon and Highly Active Transition Metals (Fe, Co, Ni) as Bifunctional Oxygen Catalysts for Hybrid Lithium-Air Batteries. Chem. Mater. 2017, 29, 1665–1675. [Google Scholar] [CrossRef]
- Su, Y.; Zhu, Y.; Jiang, H.; Shen, J.; Yang, X.; Zou, W.; Chen, J.; Li, C. Cobalt nanoparticles embedded in N-doped carbon as an efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions. Nanoscale 2014, 6, 15080–15089. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Quan, X.; Chen, S.; Liu, Y.M.; Yu, H.T. Cobalt Nanoparticles Encapsulated in Porous Carbons Derived from Core-Shell ZIF67@ZIF8 as Efficient Electrocatalysts for Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 28685–28694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, X.; Cao, X.; Zhang, B.; Tiep, N.H.; He, H.; Chen, S.; Huang, Y.; Fan, H.J. Ultrafine Metal Nanoparticles/N-Doped Porous Carbon Hybrids Coated on Carbon Fibers as Flexible and Binder-Free Water Splitting Catalysts. Adv. Energy Mater. 2017, 7, 1700220. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.; Wang, H.; Liang, Y.; Wu, J.Z.; Zhou, J.; Wang, J.; Regier, T.; Wei, F.; Dai, H. An Advanced Ni–Fe Layered Double Hydroxide Electrocatalyst for Water Oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhou, H.Q.; Sun, J.Y.; Qin, F.; Yu, F.; Bao, J.M.; Yu, Y.; Chen, S.; Ren, Z.F. Cu nanowires shelled with NiFe layered double hydroxide nanosheets as bifunctional electrocatalysts for overall water splitting. Energy Environ. Sci. 2017, 10, 1820–1827. [Google Scholar] [CrossRef]
- Song, Z.S.; Han, X.P.; Deng, Y.D.; Zhao, N.Q.; Hu, W.B.; Zhong, C. Clarifying the Controversial Catalytic Performance of Co(OH)2 and Co3O4 for Oxygen Reduction/Evolution Reactions Toward Efficient Zn-Air Batteries. ACS Appl. Mater. Interfaces 2017, 9, 22694–22703. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Metal-organic framework derived hybrid Co3O4-carbon porous nanowire arrays as reversible oxygen evolution electrodes. J. Am. Chem. Soc. 2014, 136, 13925–13931. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zhang, X.; Fan, B.; Zhang, J.; Zhou, M.; Yang, W.; Hu, X.; Wang, H.; Pan, B.; Xie, Y. Ultrathin Spinel-Structured Nanosheets Rich in Oxygen Deficiencies for Enhanced Electrocatalytic Water Oxidation. Angew. Chem. Int. Ed. 2015, 54, 7399–7404. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Dong, C.-L.; Hu, Z.; Huang, Y.-C.; Chen, J.-L.; Tao, L.; Yan, D.; Chen, D.; Shen, S.; Chou, S.; et al. Atomic-Scale CoOx Species in Metal-Organic Frameworks for Oxygen Evolution Reaction. Adv. Funct. Mater. 2017, 27, 1702546. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Xiong, D.; Petrovykh, D.Y.; Liu, L. Bifunctional Nickel Phosphide Nanocatalysts Supported on Carbon Fiber Paper for Highly Efficient and Stable Overall Water Splitting. Adv. Funct. Mater. 2016, 26, 4067–4077. [Google Scholar] [CrossRef]
- Faber, M.S.; Dziedzic, R.; Lukowski, M.A.; Kaiser, N.S.; Ding, Q.; Jin, S. High-performance electrocatalysis using metallic cobalt pyrite (CoS2) micro- and nanostructures. J. Am. Chem. Soc. 2014, 136, 10053–10061. [Google Scholar] [CrossRef] [PubMed]
- Han, X.P.; Wu, X.Y.; Zhong, C.; Deng, Y.D.; Zhao, N.Q.; Hu, W.B. NiCo2S4 nanocrystals anchored on nitrogen-doped carbon nanotubes as a highly efficient bifunctional electrocatalyst for rechargeable zinc-air batteries. Nano Energy 2017, 31, 541–550. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Pohl, D.; Rellinghaus, B.; Dong, R.; Liu, S.; Zhuang, X.; Feng, X. Interface Engineering of MoS2/Ni3S2 Heterostructures for Highly Enhanced Electrochemical Overall-Water-Splitting Activity. Angew. Chem. Int. Ed. 2016, 55, 6702–6707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Park, M.; Zhou, L.M.; Lee, G.H.; Shin, J.; Hu, Z.; Chou, S.L.; Chen, J.; Kang, Y.M. Cobalt-Doped FeS2 Nanospheres with Complete Solid Solubility as a High-Performance Anode Material for Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2016, 55, 12822–12826. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jin, J.; Zhang, J. NiCo2S4@graphene as a Bifunctional Electrocatalyst for Oxygen Reduction and Evolution Reactions. ACS Appl. Mater. Interfaces 2013, 5, 5002–5008. [Google Scholar] [CrossRef] [PubMed]
- Fominykh, K.; Chernev, P.; Zaharieva, I.; Sicklinger, J.; Stefanic, G.; Döblinger, M.; Müller, A.; Pokharel, A.; Böcklein, S.; Scheu, C.; et al. Iron-Doped Nickel Oxide Nanocrystals as Highly Efficient Electrocatalysts for Alkaline Water Splitting. ACS Nano 2015, 9, 5180–5188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Guan, B.; Gu, J.N.; Li, Y.; Ma, C.; Zhao, J.; Wang, T.Y.; Cheng, C.J. One-step synthesis of nickel cobalt sulphides particles: Tuning the composition for high performance supercapacitors. RSC Adv. 2016, 6, 58916–58924. [Google Scholar] [CrossRef]
- Chen, H.C.; Jiang, J.J.; Zhao, Y.D.; Zhang, L.; Guo, D.Q.; Xia, D.D. One-pot synthesis of porous nickel cobalt sulphides: Tuning the composition for superior pseudocapacitance. J. Mater. Chem. A 2015, 3, 428–437. [Google Scholar] [CrossRef]
- Cui, Y.; Zhou, C.W.; Li, X.Z.; Gao, Y.; Zhang, J. High performance electrocatalysis for hydrogen evolution reaction using nickel-doped CoS2 nanostructures: Experimental and DFT insights. Electrochim. Acta 2017, 228, 428–435. [Google Scholar] [CrossRef]
- Kong, D.; Cha, J.J.; Wang, H.; Lee, H.R.; Cui, Y. First-row transition metal dichalcogenide catalysts for hydrogen evolution reaction. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar] [CrossRef]
- Chen, G.; Liaw, S.S.; Li, B.; Xu, Y.; Dunwell, M.; Deng, S.; Fan, H.; Luo, H. Microwave-assisted synthesis of hybrid CoxNi1−x(OH)2 nanosheets: Tuning the composition for high performance supercapacitor. J. Power Sources 2014, 251, 338–343. [Google Scholar] [CrossRef]
- Yang, G.; Zhong, H.; Liu, R.; Li, Y.; Zou, B. In Situ Aggregation of ZnSe Nanoparticles into Supraparticles: Shape Control and Doping Effects. Langmuir 2013, 29, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Lambert, T.N.; Vigil, J.A.; White, S.E.; Davis, D.J.; Limmer, S.J.; Burton, P.D.; Coker, E.N.; Beechem, T.E.; Brumbach, M.T. Electrodeposited NixCo3−xO4 nanostructured films as bifunctional oxygen electrocatalysts. Chem. Commun. 2015, 51, 9511–9514. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, C.; Sumboja, A.; Lee, P.S. High performance porous nickel cobalt oxide nanowires for asymmetric supercapacitor. Nano Energy 2014, 3, 119–126. [Google Scholar] [CrossRef]
- Peng, Z.; Jia, D.S.; Al-Enizi, A.M.; Elzatahry, A.A.; Zheng, G.F. From Water Oxidation to Reduction: Homologous Ni-Co Based Nanowires as Complementary Water Splitting Electrocatalysts. Adv. Energy Mater. 2015, 5, 1402031. [Google Scholar] [CrossRef]
- Vrubel, H.; Merki, D.; Hu, X. Hydrogen evolution catalyzed by MoS3 and MoS2 particles. Energy Environ. Sci. 2012, 5, 6136–6144. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Lin, C.-T.; Chen, T.-Y.; Hsu, C.-L.; Lee, Y.-H.; Zhang, W.; Wei, K.-H.; Li, L.-J. Highly Efficient Electrocatalytic Hydrogen Production by MoSx Grown on Graphene-Protected 3D Ni Foams. Adv. Mater. 2013, 25, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wan, L.; Yang, S.; Xiao, F.; Wang, S. Design Hierarchical Electrodes with Highly Conductive NiCo2S4 Nanotube Arrays Grown on Carbon Fiber Paper for High-Performance Pseudocapacitors. Nano Lett. 2014, 14, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Sivanantham, A.; Ganesan, P.; Shanmugam, S. Hierarchical NiCo2S4 Nanowire Arrays Supported on Ni Foam: An Efficient and Durable Bifunctional Electrocatalyst for Oxygen and Hydrogen Evolution Reactions. Adv. Funct. Mater. 2016, 26, 4661–4672. [Google Scholar] [CrossRef]
- Appandairajan, N.K.; Gopalakrishnan, J. A study of Co3−xNixO4 (O ≤ x ≤ 1) system. Proc. Indian Acad. Sci. Sect. A 1978, 87, 115–120. [Google Scholar]








| Catalysts | Reaction | Tafel Slope (mV dec−1) | Overpotential (mV) HER@30 mA cm−2 OER@50 mA cm−2 | Rct (Ω) | Cdl (mF cm−2) |
|---|---|---|---|---|---|
| CoS2 | HER | 98 | 410 | 7.2 | |
| OER | 99 | 340 | |||
| Ni0.33Co0.67S2 | HER | 76 | 350 | 3.3 | 24.1 |
| OER | 55 | 286 | |||
| Ni0.5Co0.5S2 | HER | 82 | 370 | 3.9 | 17.6 |
| OER | 61 | 292 | |||
| Ni0.67Co0.33S2 | HER | 89 | 387 | 4.5 | 9.5 |
| OER | 68 | 297 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, G.; Zhang, W.; Deng, Y.; Zhong, C.; Hu, W.; Han, X. Engineering Pyrite-Type Bimetallic Ni-Doped CoS2 Nanoneedle Arrays over a Wide Compositional Range for Enhanced Oxygen and Hydrogen Electrocatalysis with Flexible Property. Catalysts 2017, 7, 366. https://doi.org/10.3390/catal7120366
He G, Zhang W, Deng Y, Zhong C, Hu W, Han X. Engineering Pyrite-Type Bimetallic Ni-Doped CoS2 Nanoneedle Arrays over a Wide Compositional Range for Enhanced Oxygen and Hydrogen Electrocatalysis with Flexible Property. Catalysts. 2017; 7(12):366. https://doi.org/10.3390/catal7120366
Chicago/Turabian StyleHe, Guowei, Wei Zhang, Yida Deng, Cheng Zhong, Wenbin Hu, and Xiaopeng Han. 2017. "Engineering Pyrite-Type Bimetallic Ni-Doped CoS2 Nanoneedle Arrays over a Wide Compositional Range for Enhanced Oxygen and Hydrogen Electrocatalysis with Flexible Property" Catalysts 7, no. 12: 366. https://doi.org/10.3390/catal7120366
APA StyleHe, G., Zhang, W., Deng, Y., Zhong, C., Hu, W., & Han, X. (2017). Engineering Pyrite-Type Bimetallic Ni-Doped CoS2 Nanoneedle Arrays over a Wide Compositional Range for Enhanced Oxygen and Hydrogen Electrocatalysis with Flexible Property. Catalysts, 7(12), 366. https://doi.org/10.3390/catal7120366