Enhanced Photodegradation Activity of Hydrogen-Terminated Si Nanowires Arrays with Different-Oriented Crystal Phases
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Si NWs
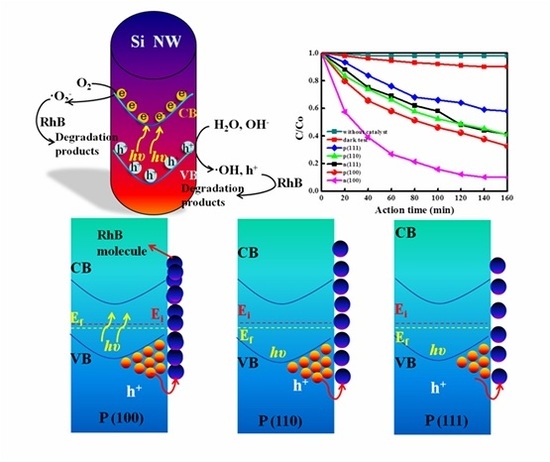
2.2. Photodegradation Activity of Si NWs with Various Crystal Orientations
3. Experimental Section
3.1. Fabrications of Si NWs Arrays with Various Crystal Orientations
3.2. RhB Degradation Process
3.3. Characterizations of Si NWs Arrays with Various Crystal Orientation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liao, F.; Wang, T.; Shao, M. Silicon nanowires: Applications in catalysis with distinctive surface property. J. Mater. Sci. Mater. Electr. 2015, 26, 4722–4729. [Google Scholar] [CrossRef]
- Yuan, G.D.; Zhou, Y.B.; Guo, C.S.; Zhang, W.J.; Tang, Y.B.; Li, Y.Q.; Chen, Z.H.; He, Z.B.; Zhang, X.J.; Wang, P.F. Tunable electrical properties of silicon nanowires via surface-ambient chemistry. ACS Nano 2010, 4, 3045–3052. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.F.; Ruffo, R.; Chan, C.K.; Peng, H.; Cui, Y. Crystalline-amorphous core-shell silicon nanowires for high capacity and high current battery electrodes. Nano Lett. 2009, 9, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Dr, K.P.; Xu, Y.; Wu, Y.; Yan, Y.; Lee, S.T.; Jing, Z.P. Aligned single-crystalline Si nanowire arrays for photovoltaic applications. Small 2005, 1, 1062–1067. [Google Scholar]
- Holmes, J.D.; Johnston, K.P.; Doty, R.C.; Korgel, B.A. Control of thickness and orientation of solution-grown silicon nanowires. Science 2000, 287, 1471–1473. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Peng, H.; Liu, G.; Mcilwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, W.; Lim, J.; Yu, Y.; Kong, Q.; Urban, J.J.; Yang, P. Thermal transport in silicon nanowires at high temperature up to 700 K. Nano Lett. 2016, 16, 4133–4140. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Niquet, Y.M.; Delerue, C. Complexity of the hot carrier relaxation in Si nanowires compared to bulk. Phys. Rev. B 2017, 95. [Google Scholar] [CrossRef]
- Knopfmacher, O.; Tarasov, A.; Fu, W.; Wipf, M.; Niesen, B.; Calame, M.; Schönenberger, C. Nernst limit in Dual-Gated Si-nanowire FET Sensors. Nano Lett. 2010, 10, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Lu, W.; Hu, Y.; Wu, Y.; Yan, H.; Lieber, C.M. Ge/Si NW heterostructures as high-performance field-effect transistors. Nature 2006, 441, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Hochbaum, A.I.; Fan, R.; He, R.; Yang, P. Controlled growth of Si nanowire arrays for device integration. Nano Lett. 2005, 5, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Lauhon, L.J.; Gudiksen, M.S.; Wang, D.; Lieber, C.M. Epitaxial core-shell and core-multishell nanowire heterostructures. Nature 2002, 420, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Tsakalakos, L.; Balch, J.; Fronheiser, J.; Korevaar, B.A.; Sulima, O.; Rand, J. Silicon nanowire solar cells. Appl. Phys. Lett. 2007, 91, 692. [Google Scholar] [CrossRef]
- Tian, B.; Zheng, X.; Kempa, T.J.; Fang, Y.; Yu, N.; Yu, G.; Huang, J.; Lieber, C.M. Coaxial silicon nanowires as solar cells and nanoelectronic power sources. Nature 2007, 449, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Garnett, E.; Yang, P. Light trapping in silicon nanowire solar cells. Nano Lett. 2010, 10, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, J.; Sun, T.; Wang, M.; Wu, C.; Zou, H.; Song, T.; Zhang, X.; Lee, S.T.; Wang, Z.L. Silicon nanowire/polymer hybrid solar cell-supercapacitor: A self-charging power unit with a total efficiency of 10.5. Nano Lett. 2017, 17, 4240–4247. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wu, J.; Liu, S.; Xiong, J.; Jagadish, C.; Wang, Z.M. Design and fabrication of silicon nanowires towards efficient solar cells. Nano Today 2016, 11, 704–737. [Google Scholar] [CrossRef]
- Ghosh, R.; Imakita, K.; Fujii, M.; Giri, P.K. Effect of Ag/Au bilayer assisted etching on the strongly enhanced photoluminescence and visible light photocatalysis by Si nanowire arrays. Phys. Chem. Chem. Phys. 2016, 18, 7715–7727. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.; Aspetti, C.O.; Cargnello, M.; Ren, M.L.; Yoo, J.; Murray, C.B.; Agarwal, R. Engineering localized surface plasmon interactions in gold by silicon nanowire for enhanced heating and photocatalysis. Nano Lett. 2017, 17, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Meysami, S.S.; Babenko, V.; Huang, C.; Luanwuthi, S.; Acapulco, J.; Holdway, P.; Grant, P.S.; Grobert, N. Vertically-aligned silicon carbide nanowires as visible-light-driven photocatalysts. Appl. Catal. B Environ. 2017, 218, 267–276. [Google Scholar] [CrossRef]
- Wang, F.Y.; Yang, Q.D.; Xu, G.; Lei, N.Y.; Tsang, Y.K.; Wong, N.B.; Ho, J.C. Highly active and enhanced photocatalytic silicon nanowire arrays. Nanoscale 2011, 3, 3269–3276. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Cheng, L.; Zhang, X.; Ma, D.D.; Lee, S.T. Excellent photocatalysis of HF-treated silicon nanowires. J. Am. Chem. Soc. 2009, 131, 17738–17739. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.; Kye, J.; Hwang, S. Enhanced photoelectrochemical hydrogen production from silicon nanowire array photocathode. Nano Lett. 2012, 12, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.J.; Boukai, A.; Yang, P. High density n-Si/n-TiO2 core/shell nanowire arrays with enhanced photoactivity. Nano Lett. 2009, 9, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, H.; Zhang, X.H.; Lee, C.S.; Lee, S.T. Wafer-scale synthesis of single-crystal zigzag silicon nanowire arrays with controlled turning angles. Nano Lett. 2010, 10, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Megouda, N.; Cofininier, Y.; Szunerits, S.; Hadjersi, T.; Elkechai, O.; Boukherroub, R. Photocatalytic activity of silicon nanowires under UV and visible light irradiation. Chem. Commun. 2011, 47, 991–993. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, L.; Gao, Y.; Wang, C.; Jiang, J.; Xiong, Y. The nature of photocatalytic “water splitting” on silicon nanowires. Angew. Chem. 2015, 54, 2980–2985. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Tang, Y.B.; Liu, Y.; Kang, Z.H. Dye degradation induced by hydrogen-terminated silicon nanowires under ultrasonic agitations. J. Appl. Phys. 2009, 105, 1289. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hsiao, P.H.; Wei, T.C.; Chen, T.C.; Tang, C.H. Well incorporation of carbon nanodots with silicon nanowire arrays featuring excellent photocatalytic performances. Phys. Chem. Chem. Phys. 2017, 19, 11786–11792. [Google Scholar] [CrossRef] [PubMed]
- Brahiti, N.; Hadjersi, T.; Menari, H.; Amirouche, S.; Kechai, O.E. Enhanced photocatalytic degradation of methylene blue by metal-modified silicon nanowires. Mater. Res. Bull. 2015, 62, 30–36. [Google Scholar] [CrossRef]
- Hasan, M.; Huq, M.F.; Mahmood, Z.H. A review on electronic and optical properties of silicon nanowire and its different growth techniques. Springerplus 2013, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Ducati, C.; Neill, R.J.; Piscanec, S.; Ferrari, A.C.; Geng, J.; Duninborkowski, R.E.; Robertson, J. Gold catalyzed growth of silicon nanowires by plasma enhanced chemical vapor deposition. J. Appl. Phys. 2003, 94, 6005–6012. [Google Scholar] [CrossRef]
- Alet, P.J.; Yu, L.; Patriarche, G.; Palacin, S.; Cabarrocas, P.R.I. In situ generation of indium catalysts to grow crystalline silicon nanowires at low temperature on ITO. J. Mater. Chem. 2008, 18, 5187–5189. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.Y.; Reuter, M.C.; Tersoff, J.; Stach, E.A.; Ross, F.M. Structure, growth kinetics, and ledge flow during vapor-solid-solid growth of copper-catalyzed silicon nanowires. Nano Lett. 2013, 10, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cui, Y.; Huynh, L.; Barrelet, C.J.; David, C.B.; Liber, C.M. Controlled growth and structures of molecular-scale silicon nanowires. Nano Lett. 2004, 4, 433–436. [Google Scholar] [CrossRef]
- Schmidt, V.; Senz, S.; Gösele, U. Diameter-dependent growth direction of epitaxial silicon nanowires. Nano Lett. 2005, 5, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.M.; Yuyama, Y.; Sato, T.; Fujikawa, S.; Uozumi, Y. A palladium-nanoparticle and silicon-nanowire-array hybrid: A platform for catalytic heterogeneous reactions. Angew. Chem. 2014, 53, 131–135. [Google Scholar] [CrossRef]
- Huang, Z.; Geyer, N.; Werner, P.; De Boor, J.; Gösele, U. Metal-assisted chemical etching of silicon: A review. Adv. Mater. 2011, 23, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Shimizu, T.; Senz, S.; Zhang, Z.; Zhang, X.; Lee, W.; Geyer, N.; Gösele, U. Ordered arrays of vertically aligned [110] silicon nanowires by suppressing the crystallographically preferred etching directions. Nano Lett. 2009, 9, 2519–2525. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xiao, H.; Pei, H.; Cui, J.; Hu, W. Photodegradation activity and stability of porous silicon wafers with (100) and (111) oriented crystal planes. Microporous Mesoporous Mater. 2015, 204, 251–256. [Google Scholar] [CrossRef]
- Prince, M.B. Drift Mobilities in Semiconductors. II. Silicon. Phys. Rev. 1954, 93, 1204–1206. [Google Scholar] [CrossRef]
- Ansari, D.M.; Price, G.J. Chromatographic estimation of filler surface energies and correlation with photodegradation of kaolin filled polyethylene. Polymer 2004, 45, 1823–1831. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Chou, M.Y. Effect of hydrogen on the surface-energy anisotropy of diamond and silicon. Phys. Rev. B 1998, 57, 6262–6265. [Google Scholar] [CrossRef]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Luo, L.; Song, J.; Zhang, H.; Li, X.; Cheng, S.; Jin, W.; Tang, J.; Liu, L.; Wang, F. Enhanced Photodegradation Activity of Hydrogen-Terminated Si Nanowires Arrays with Different-Oriented Crystal Phases. Catalysts 2017, 7, 371. https://doi.org/10.3390/catal7120371
Song L, Luo L, Song J, Zhang H, Li X, Cheng S, Jin W, Tang J, Liu L, Wang F. Enhanced Photodegradation Activity of Hydrogen-Terminated Si Nanowires Arrays with Different-Oriented Crystal Phases. Catalysts. 2017; 7(12):371. https://doi.org/10.3390/catal7120371
Chicago/Turabian StyleSong, Longfei, Linqu Luo, Jianjun Song, Hongchao Zhang, Xuan Li, Shuiling Cheng, Wei Jin, Jie Tang, Lei Liu, and Fengyun Wang. 2017. "Enhanced Photodegradation Activity of Hydrogen-Terminated Si Nanowires Arrays with Different-Oriented Crystal Phases" Catalysts 7, no. 12: 371. https://doi.org/10.3390/catal7120371
APA StyleSong, L., Luo, L., Song, J., Zhang, H., Li, X., Cheng, S., Jin, W., Tang, J., Liu, L., & Wang, F. (2017). Enhanced Photodegradation Activity of Hydrogen-Terminated Si Nanowires Arrays with Different-Oriented Crystal Phases. Catalysts, 7(12), 371. https://doi.org/10.3390/catal7120371