Photocatalytic TiO2 Nanorod Spheres and Arrays Compatible with Flexible Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Diffraction and Raman Spectroscopy
2.2. Electron Microscopy
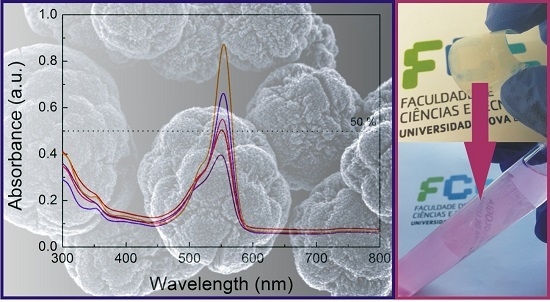
2.3. Optical Characterization
2.4. Photocatalytic Activity
3. Experimental
3.1. TiO2 Nanorod Sphere and Array Productions
3.2. Structural and Optical Characterizations
3.3. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interests
References
- Bai, J.; Zhou, B. Titanium dioxide nanomaterials for sensor applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef] [PubMed]
- Bernacka-Wojcik, I.; Senadeera, R.; Wojcik, P.J.; Silva, L.B.; Doria, G.; Baptista, P.; Aguas, H.; Fortunato, E.; Martins, R. Inkjet printed and “doctor blade” TiO2 photodetectors for DNA biosensors. Biosens. Bioelectron. 2010, 25, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Heo, Y.-U.; Nattestad, A.; Sun, Z.; Wang, L.; Kim, J.H.; Dou, S.X. 3D hierarchical rutile TiO2 and metal-free organic sensitizer producing dye-sensitized solar cells 8.6% conversion efficiency. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.; Nunes, D.; Pereira, S.; Martins, R.; Fortunato, E. Photocatalytic activity of TiO2 nanostructured arrays prepared by microwave-assisted solvothermal method. In Semiconductor Photocatalysis—Materials, Mechanisms and Applications; Cao, W., Ed.; InTech: Rijeka, Croatia, 2016. [Google Scholar]
- Nunes, D.; Pimentel, A.; Pinto, J.V.; Calmeiro, T.R.; Nandy, S.; Barquinha, P.; Pereira, L.; Carvalho, P.A.; Fortunato, E.; Martins, R. Photocatalytic behavior of TiO2 films synthesized by microwave irradiation. Catal. Today 2016, 278, 262–270. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xu, J.; Wang, X.; Xie, Z.; Liu, Z.; Liang, B.; Chen, D.; Shen, G. Flexible TiO2/cellulose acetate hybrid film as a recyclable photocatalyst. RSC Adv. 2014, 4, 12640–12648. [Google Scholar] [CrossRef]
- Sunada, K.; Kikuchi, Y.; Hashimoto, K.; Fujishima, A. Bactericidal and detoxification effects of TiO2 thin film photocatalysts. Environ. Sci. Technol. 1998, 32, 726–728. [Google Scholar] [CrossRef]
- Vukoje, I.; Kovač, T.; Džunuzović, J.; Džunuzović, E.; Lončarević, D.; Ahrenkiel, S.P.; Nedeljković, J.M. Photocatalytic ability of visible-light-responsive TiO2 nanoparticles. J. Phys. Chem. C 2016, 120, 18560–18569. [Google Scholar] [CrossRef]
- Kerkez, Ö.; Boz, İ. Efficient removal of methylene blue by photocatalytic degradation with TiO2 nanorod array thin films. React. Kinet. Mech. Catal. 2013, 110, 543–557. [Google Scholar] [CrossRef]
- Chen, D.Y.; Tsao, C.C.; Hsu, C.Y. Photocatalytic TiO2 thin films deposited on flexible substrates by radio frequency (RF) reactive magnetron sputtering. Curr. Appl. Phys. 2012, 12, 179–183. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Chen, J.; Wu, X.; Zhou, L. TiO2 mesocrystals built of nanocrystals with exposed {001} facets: Facile synthesis and superior photocatalytic ability. J. Mater. Chem. A 2014, 2, 19589–19593. [Google Scholar] [CrossRef]
- Kenanakis, G.; Vernardou, D.; Dalamagkas, A.; Katsarakis, N. Photocatalytic and electrooxidation properties of TiO2 thin films deposited by sol–gel. Catal. Today 2015, 240, 146–152. [Google Scholar] [CrossRef]
- Kazuhito, H.; Hiroshi, I.; Akira, F. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar]
- Rathee, D.; Arya, S.; Kumar, M. Analysis of TiO2 for microelectronic applications: Effect of deposition methods on their electrical properties. Front. Optoelectron. China 2011, 4, 349–358. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the least known TiO2 photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.; Cab, C.; De Coss, R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Konishi, Y.; Tada, H.; Tohge, N.; Nishii, J.; Ito, S. A patterned TiO2(anatase)/TiO2 (rutile) bilayer-type photocatalyst: Effect of the anatase/rutile junction on the photocatalytic activity. Angew. Chem. 2002, 114, 2935–2937. [Google Scholar] [CrossRef]
- Wang, R.; Cai, X.; Shen, F. Preparation of TiO2 hollow microspheres by a novel vesicle template method and their enhanced photocatalytic properties. Ceram. Int. 2013, 39, 9465–9470. [Google Scholar] [CrossRef]
- Andersson, M.; Österlund, L.; Ljungström, S.; Palmqvist, A. Preparation of nanosize anatase and rutile TiO2 by hydrothermal treatment of microemulsions and their activity for photocatalytic wet oxidation of phenol. J. Phys. Chem. B 2002, 106, 10674–10679. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Mori, T.; Shen, Q.; Toyoda, T. Photoluminescence study of mixtures of anatase and rutile TiO2 nanoparticles: Influence of charge transfer between the nanoparticles on their photoluminescence excitation bands. Chem. Phys. Lett. 2005, 409, 81–84. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Robben, L.; Alkaim, A.; Bahnemann, D. Brookite versus anatase TiO2 photocatalysts: Phase transformations and photocatalytic activities. Photochem. Photobiol. Sci. 2013, 12, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Boppella, R.; Basak, P.; Manorama, S.V. Viable method for the synthesis of biphasic TiO2 nanocrystals with tunable phase composition and enabled visible-light photocatalytic performance. ACS Appl. Mater. Int. 2012, 4, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Rochkind, M.; Pasternak, S.; Paz, Y. Using dyes for evaluating photocatalytic properties: A critical review. Molecules 2014, 20, 88–110. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, M.; Choung, S.-J.; Ogino, K.; Miyata, S.; Kim, M.-S.; Park, J.-Y.; Kim, J.-B. The preparation of TiO2 nanometer photocatalyst film by a hydrothermal method and its sterilization performance for giardia lamblia. Water Res. 2004, 38, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, I.; Jha, H.; Liu, N.; Schmuki, P. A review of photocatalysis using self-organized TiO2 nanotubes and other ordered oxide nanostructures. Small 2012, 8, 3073–3103. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Sato, K.; Takami, S.; Numako, C.; Umetsu, M.; Soga, K.; Nakayama, M.; Sasaki, R.; Tanaka, T.; Ogino, C.; et al. Particle size for photocatalytic activity of anatase TiO2 nanosheets with highly exposed {001} facets. RSC Adv. 2013, 3, 19268–19271. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, D.; Tang, G.; Ji, X.; Li, C.; Yan, X.; Wu, Q. Low temperature synthesis and photocatalytic properties of mesoporous TiO2 nanospheres. J. Alloys Compd. 2014, 591, 52–57. [Google Scholar] [CrossRef]
- Hafez, H.S. Synthesis of highly-active single-crystalline TiO2 nanorods and its application in environmental photocatalysis. Mater. Lett. 2009, 63, 1471–1474. [Google Scholar] [CrossRef]
- Scuderi, V.; Impellizzeri, G.; Zimbone, M.; Sanz, R.; Di Mauro, A.; Buccheri, M.A.; Miritello, M.; Terrasi, A.; Rappazzo, G.; Nicotra, G.; et al. Rapid synthesis of photoactive hydrogenated TiO2 nanoplumes. Appl. Catal. B Environ. 2016, 183, 328–334. [Google Scholar] [CrossRef]
- Jitputti, J.; Suzuki, Y.; Yoshikawa, S. Synthesis of TiO2 nanowires and their photocatalytic activity for hydrogen evolution. Catal. Commun. 2008, 9, 1265–1271. [Google Scholar] [CrossRef]
- Bai, H.; Liu, Z.; Liu, L.; Sun, D.D. Large-scale production of hierarchical TiO2 nanorod spheres for photocatalytic elimination of contaminants and killing bacteria. Chem. Eur. J. 2013, 19, 3061–3070. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Y.; Chen, B.-R. Characterization and photocatalytic activity of TiO2 nanotube films prepared by anodization. Int. J. Photoenergy 2013, 2013. [Google Scholar] [CrossRef]
- Xingtao, J.; Wen, H.; Xudong, Z.; Hongshi, Z.; Zhengmao, L.; Yingjun, F. Microwave-assisted synthesis of anatase TiO2 nanorods with mesopores. Nanotechnology 2007, 18, 075602. [Google Scholar]
- Gregori, D.; Benchenaa, I.; Chaput, F.; Therias, S.; Gardette, J.L.; Leonard, D.; Guillard, C.; Parola, S. Mechanically stable and photocatalytically active TiO2/SiO2 hybrid films on flexible organic substrates. J. Mater. Chem. A 2014, 2, 20096–20104. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, D.M.; Ying, J.Y. Synthesis of hexagonally packed mesoporous TiO2 by a modified sol–gel method. Angew. Chem. Int. Ed. Engl. 1995, 34, 2014–2017. [Google Scholar] [CrossRef]
- Scuderi, V.; Impellizzeri, G.; Romano, L.; Scuderi, M.; Nicotra, G.; Bergum, K.; Irrera, A.; Svensson, B.G.; Privitera, V. TiO2-coated nanostructures for dye photo-degradation in water. Nanoscale Res. Lett. 2014, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-M.; Shih, H.C.; Wu, W.-T.; Tseng, Y.-K.; Chen, I.C. Thermal evaporation growth and the luminescence property of TiO2 nanowires. J. Cryst. Growth 2005, 281, 384–390. [Google Scholar] [CrossRef]
- Boyadzhiev, S.; Georgieva, V.; Rassovska, M. Characterization of reactive sputtered TiO2 thin films for gas sensor applications. J. Phys. 2010, 253, 012040. [Google Scholar]
- Lee, D.; Rho, Y.; Allen, F.I.; Minor, A.M.; Ko, S.H.; Grigoropoulos, C.P. Synthesis of hierarchical TiO2 nanowires with densely-packed and omnidirectional branches. Nanoscale 2013, 5, 11147–11152. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, Y.; Xiao, W. Enhanced photoelectrocatalytic performance of SnO2/TiO2 rutile composite films. J. Mater. Chem. A 2013, 1, 10727–10735. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, M.; Zhu, Y. Fabrication of porous TiO2 film via hydrothermal method and its photocatalytic performances. Thin Solid Films 2007, 515, 7127–7134. [Google Scholar] [CrossRef]
- Chen, Q.; Qian, Y.; Chen, Z.; Wu, W.; Chen, Z.; Zhou, G.; Zhang, Y. Hydrothermal epitaxy of highly oriented TiO2 thin films on silicon. Appl. Phys. Lett. 1995, 66, 1608–1610. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, J.-J.; Hong, J.-M.; Bian, N.; Chen, H.-Y. Microwave-induced polyol-process synthesis of copper and copper oxide nanocrystals with controllable morphology. Eur. J. Inorg. Chem. 2004, 2004, 4072–4080. [Google Scholar] [CrossRef]
- Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave assisted organic synthesis—A review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Barquinha, P.; Carvalho, P.A.; Fortunato, E.; Martins, R. Cu2O polyhedral nanowires produced by microwave irradiation. J. Mater. Chem. C 2014, 2, 6097–6103. [Google Scholar] [CrossRef]
- Yang, M.; Ding, B.; Lee, S.; Lee, J.-K. Carrier transport in dye-sensitized solar cells using single crystalline TiO2 nanorods grown by a microwave-assisted hydrothermal reaction. J. Phys. Chem. C 2011, 115, 14534–14541. [Google Scholar] [CrossRef]
- Pimentel, A.; Rodrigues, J.; Duarte, P.; Nunes, D.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Effect of solvents on ZnO nanostructures synthesized by solvothermal method assisted by microwave radiation: A photocatalytic study. J. Mater. Sci. 2015, 50, 5777–5787. [Google Scholar] [CrossRef]
- Pimentel, A.; Nunes, D.; Duarte, P.; Rodrigues, J.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Synthesis of long ZnO nanorods under microwave irradiation or conventional heating. J. Phys. Chem. C 2014, 118, 14629–14639. [Google Scholar] [CrossRef]
- Marques, A.C.; Santos, L.; Costa, M.N.; Dantas, J.M.; Duarte, P.; Gonçalves, A.; Martins, R.; Salgueiro, C.A.; Fortunato, E. Office paper platform for bioelectrochromic detection of electrochemically active bacteria using tungsten trioxide nanoprobes. Sci. Rep. 2015, 5, 9910. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Resende, J.; Marques, A.C.; Pinto, J.V.; Nunes, D.; Marie, A.; Goncalves, R.; Pereira, L.; Martins, R.; Fortunato, E. Smart optically active VO2 nanostructured layers applied in roof-type ceramic tiles for energy efficiency. Sol. Energy Mater. Sol. C 2016, 150, 1–9. [Google Scholar] [CrossRef]
- Herring, N.P.; Panda, A.B.; AbouZeid, K.; Almahoudi, S.H.; Olson, C.R.; Patel, A.; El-Shall, M.S. Microwave synthesis of metal oxide nanoparticles. In Metal Oxide Nanomaterials for Chemical Sensors; Carpenter, A.M., Mathur, S., Kolmakov, A., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Wang, Y.; Zhang, L.; Deng, K.; Chen, X.; Zou, Z. Low temperature synthesis and photocatalytic activity of rutile TiO2 nanorod superstructures. J. Phys. Chem. C 2007, 111, 2709–2714. [Google Scholar] [CrossRef]
- Yan, J.; Feng, S.; Lu, H.; Wang, J.; Zheng, J.; Zhao, J.; Li, L.; Zhu, Z. Alcohol induced liquid-phase synthesis of rutile titania nanotubes. Mater. Sci. Eng. B 2010, 172, 114–120. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Huang, X.; Li, Q.; Li, G. New insights into fluorinated TiO2 (brookite, anatase and rutile) nanoparticles as efficient photocatalytic redox catalysts. RSC Adv. 2015, 5, 34302–34313. [Google Scholar] [CrossRef]
- Wang, M.; Li, Q.; Yu, H.; Hur, S.H.; Kim, E.J. Phase-controlled preparation of TiO2 films and micro(nano)spheres by low-temperature chemical bath deposition. J. Alloys Compd. 2013, 578, 419–424. [Google Scholar] [CrossRef]
- Hayes, B.L. Microwave Synthesis: Chemistry at the Speed of Light; CEM Pub.: Matthews, NC, USA, 2002. [Google Scholar]
- Cao, G.; Wang, Y. Nanostructures and Nanomaterials: Synthesis, Properties, and Applications; World Scientific: Singapore, 2011. [Google Scholar]
- Zhou, W.; Liu, X.; Cui, J.; Liu, D.; Li, J.; Jiang, H.; Wang, J.; Liu, H. Control synthesis of rutile TiO2 microspheres, nanoflowers, nanotrees and nanobelts via acid-hydrothermal method and their optical properties. CrystEngComm 2011, 13, 4557–4563. [Google Scholar] [CrossRef]
- Pimentel, A.; Ferreira, S.; Nunes, D.; Calmeiro, T.; Martins, R.; Fortunato, E. Microwave synthesized ZnO nanorod arrays for UV sensors: A seed layer annealing temperature study. Material 2016, 9, 299. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, M.S.; Wu, J.; Kang, L.; Yang, S.; Wu, P.; Yin, Z. Optical properties of SrTiO3 thin films by pulsed laser deposition. Appl. Phys. A 2003, 76, 1105–1108. [Google Scholar] [CrossRef]
- Aydın, C.; Benhaliliba, M.; Al-Ghamdi, A.; Gafer, Z.; El-Tantawy, F.; Yakuphanoglu, F. Determination of optical band gap of ZnO:ZnAl2O4 composite semiconductor nanopowder materials by optical reflectance method. J. Electroceram. 2013, 31, 265–270. [Google Scholar] [CrossRef]
- Yu, J.-G.; Yu, H.-G.; Cheng, B.; Zhao, X.-J.; Yu, J.C.; Ho, W.-K. The effect of calcination temperature on the surface microstructure and photocatalytic activity of TiO2 thin films prepared by liquid phase deposition. J. Phys. Chem. B 2003, 107, 13871–13879. [Google Scholar] [CrossRef]
- Wu, J.-M.; Shih, H.C.; Wu, W.-T. Formation and photoluminescence of single-crystalline rutile TiO2 nanowires synthesized by thermal evaporation. Nanotechnology 2006, 17, 105. [Google Scholar] [CrossRef]
- Nagaveni, K.; Sivalingam, G.; Hegde, M.S.; Madras, G. Solar photocatalytic degradation of dyes: High activity of combustion synthesized nano TiO2. Appl. Catal. B Environ. 2004, 48, 83–93. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Mader, E.; Ma, P.-C. Controlled synthesis of hierarchical TiO2 nanoparticles on glass fibres and their photocatalytic performance. Dalton Trans. 2014, 43, 12743–12753. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Tu, K.-J. Thickness dependent on photocatalytic activity of hematite thin films. Int. J. Photoenergy 2011, 2012. [Google Scholar] [CrossRef]
- Wang, H.; Gao, X.; Duan, G.; Yang, X.; Liu, X. Facile preparation of anatase–brookite–rutile mixed-phase N-doped TiO2 with high visible-light photocatalytic activity. J. Environ. Chem. Eng. 2015, 3, 603–608. [Google Scholar] [CrossRef]
- Qiu, S.; Ben, T. Porous Polymers: Design, Synthesis and Applications; Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar]
- Fann, D.M.; Huang, S.K.; Lee, J.Y. DSC studies on the crystallization characteristics of poly(ethylene terephthalate) for blow molding applications. Polym. Eng. Sci. 1998, 38, 265–273. [Google Scholar] [CrossRef]
- Kim, W.-Y.; Kim, S.-W.; Yoo, D.-H.; Kim, E.J.; Hahn, S.H. Annealing effect of ZnO seed layer on enhancing photocatalytic activity of ZnO/TiO2 nanostructure. Int. J. Photoenergy 2013, 2013. [Google Scholar] [CrossRef]
- Chou, H.-T.; Hsu, H.-C. The effect of annealing temperatures to prepare ZnO seeds layer on ZnO nanorods array/TiO2 nanoparticles photoanode. Solid-State Electr. 2016, 116, 15–21. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. Powder cell—A program for the representation and manipulation of crystal structures and calculation of the resulting X-Ray powder patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Pearson, W.B.; Villars, P.; Calvert, L.D. Pearson’s Handbook of Crystall Ographic Data for Intermetallic Phases; American Society for Metals: Cleveland, OH, USA, 1985. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to image J: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Santos, L.; Nunes, D.; Calmeiro, T.; Branquinho, R.; Salgueiro, D.; Barquinha, P.; Pereira, L.; Martins, R.; Fortunato, E. Solvothermal synthesis of gallium-indium-zinc-oxide nanoparticles for electrolyte-gated transistors. ACS Appl. Mater. Interface 2015, 7, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]









© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, D.; Pimentel, A.; Santos, L.; Barquinha, P.; Fortunato, E.; Martins, R. Photocatalytic TiO2 Nanorod Spheres and Arrays Compatible with Flexible Applications. Catalysts 2017, 7, 60. https://doi.org/10.3390/catal7020060
Nunes D, Pimentel A, Santos L, Barquinha P, Fortunato E, Martins R. Photocatalytic TiO2 Nanorod Spheres and Arrays Compatible with Flexible Applications. Catalysts. 2017; 7(2):60. https://doi.org/10.3390/catal7020060
Chicago/Turabian StyleNunes, Daniela, Ana Pimentel, Lidia Santos, Pedro Barquinha, Elvira Fortunato, and Rodrigo Martins. 2017. "Photocatalytic TiO2 Nanorod Spheres and Arrays Compatible with Flexible Applications" Catalysts 7, no. 2: 60. https://doi.org/10.3390/catal7020060
APA StyleNunes, D., Pimentel, A., Santos, L., Barquinha, P., Fortunato, E., & Martins, R. (2017). Photocatalytic TiO2 Nanorod Spheres and Arrays Compatible with Flexible Applications. Catalysts, 7(2), 60. https://doi.org/10.3390/catal7020060