Promotive Effect of Sn2+ on Cu0/Cu+ Ratio and Stability Evolution of Cu/SiO2 Catalyst in the Hydrogenation of Dimethyl Oxalate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterisation of the Catalysts
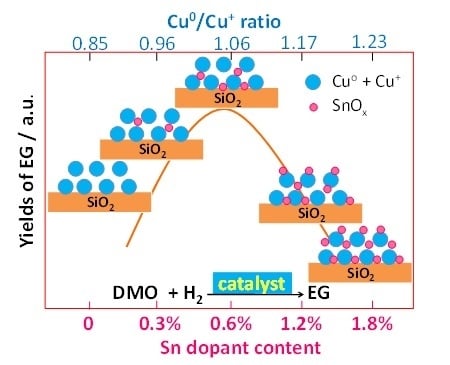
2.2. Catalytic Activity and Stability
2.3. Characterisation of the Used Catalysts
3. Experimental Section
3.1. Catalyst Preparation
3.2. Characterisation
3.3. Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Zhang, S.; Liu, Q.; Fan, G.; Li, F. Highly dispersed copper-based catalysts from Cu–Zn–Al layered double hydroxide precursor for gas-phase hydrogenation of dimethyl oxalate to ethylene glycol. Catal. Lett. 2012, 142, 1121–1127. [Google Scholar] [CrossRef]
- Chen, L.; Guo, P.; Qiao, M.; Yan, S.; Li, H.; Shen, W.; Xu, H.; Fan, K. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- Yin, A.; Guo, X.; Dai, W.-L.; Li, H.; Fan, K. Highly active and selective copper-containing HMS catalyst in the hydrogenation of dimethyl oxalate to ethylene glycol. Appl. Catal. A 2008, 349, 91–99. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Wang, S.R.; Zhu, L.J.; Ge, X.L.; Li, X.B.; Luo, Z.Y. The influence of copper particle dispersion in Cu/SiO2 catalysts on the hydrogenation synthesis of ethylene glycol. Catal. Lett. 2010, 135, 275–281. [Google Scholar] [CrossRef]
- Gong, J.; Yue, H.; Zhao, Y.; Zhao, S.; Zhao, L.; Lv, J.; Wang, S.; Ma, X. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0–Cu+ sites. J. Am. Chem. Soc. 2012, 134, 13922–13925. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lu, C.-S.; Li, X.-N. The effect of the amount of ammonia on the Cu0/Cu+ ratio of Cu/SiO2 catalyst for the hydrogenation of dimethyl oxalate to ethylene glycol. Chin. Chem. Lett. 2014, 25, 1461–1465. [Google Scholar] [CrossRef]
- He, Z.; Lin, H.; He, P.; Yuan, Y. Effect of boric oxide doping on the stability and activity of a Cu–SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2011, 277, 54–63. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, Y.; Zhao, S.; Wang, B.; Ma, X.; Gong, J. A copper-phyllosilicate core-sheath nanoreactor for carbon-oxygen hydrogenolysis reactions. Nat. Commun. 2013, 4, 2339. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-N.; Duan, X.; Zheng, J.; Lin, H.; Yuan, Y.; Ariga, H.; Takakusagi, S.; Asakura, K. Remarkable enhancement of Cu catalyst activity in hydrogenation of dimethyl oxalate to ethylene glycol using gold. Catal. Sci. Technol. 2012, 2, 1637. [Google Scholar] [CrossRef]
- Huang, Y.; Ariga, H.; Zheng, X.; Duan, X.; Takakusagi, S.; Asakura, K.; Yuan, Y. Silver-modulated SiO2-supported copper catalysts for selective hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2013, 307, 74–83. [Google Scholar] [CrossRef]
- Kaddouri, A.; Mazzocchia, C.; Tempesti, E. Sol-gel processing of copper-chromium catalysts for ester hydrogenation. J. Therm. Anal. Calorim. 1998, 53, 533–545. [Google Scholar] [CrossRef]
- Coupé, J.N.; Jordão, E.; Fraga, M.A.; Mendes, M.J. A comparative study of SiO2 supported Rh–Sn catalysts prepared by different methods in the hydrogenation of citral. Appl. Catal. A 2000, 199, 45–51. [Google Scholar]
- Luo, G.; Yan, S.; Qiao, M.; Zhuang, J.; Fan, K. Effect of tin on Ru-B/γ-Al2O3 catalyst for the hydrogenation of ethyl lactate to 1,2-propanediol. Appl. Catal. A 2004, 275, 95–102. [Google Scholar] [CrossRef]
- Vicente, A.; Lafaye, G.; Especel, C.; Marécot, P.; Williams, C.T. The relationship between the structural properties of bimetallic Pd–Sn/SiO2 catalysts and their performance for selective citral hydrogenation. J. Catal. 2011, 283, 133–142. [Google Scholar] [CrossRef]
- Esmaeili, E.; Mortazavi, Y.; Khodadadi, A.A.; Rashidi, A.M.; Rashidzadeh, M. The role of tin-promoted Pd/MWNTs via the management of carbonaceous species in selective hydrogenation of high concentration acetylene. Appl. Surf. Sci. 2012, 263, 513–522. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Zhang, H.; Zhu, Q.; Li, C.; Shan, H. Highly selective and stable NiSn/SiO2 catalyst for isobutane dehydrogenation: Effects of Sn addition. Chemcatchem 2016, 8, 3137–3145. [Google Scholar] [CrossRef]
- De Oliveira, K.; Pouilloux, Y.; Barrault, J. Selective hydrogenation of methyl oleate into unsaturated alcohols in the presence of cobalt–tin supported over zinc oxide catalysts. J. Catal. 2001, 204, 230–237. [Google Scholar] [CrossRef]
- Lin, H.; Zheng, X.; He, Z.; Zheng, J.; Duan, X.; Yuan, Y. Cu/SiO2 hybrid catalysts containing HZSM-5 with enhanced activity and stability for selective hydrogenation of dimethyl oxalate to ethylene glycol. Appl. Catal. A 2012, 445, 287–296. [Google Scholar] [CrossRef]
- Marchi, A.J.; Fierro, J.L.G.; Santamaria, J.; Monzón, A. Dehydrogenation of isopropylic alcohol on a Cu/SiO2 catalyst: A study of the activity evolution and reactivation of the catalyst. Appl. Catal. A 1996, 142, 375–386. [Google Scholar] [CrossRef]
- Yin, A.; Guo, X.; Dai, W.-L.; Fan, K. Effect of initial precipitation temperature on the structural evolution and catalytic behavior of Cu/SiO2 catalyst in the hydrogenation of dimethyloxalate. Catal. Commun. 2011, 12, 412–416. [Google Scholar] [CrossRef]
- Ma, X.; Yang, Z.; Liu, X.; Tan, X.; Ge, Q. Dynamic redox cycle of Cu0 and Cu+ over Cu/SiO2 catalyst in ester hydrogenation. RSC Adv. 2015, 5, 37581. [Google Scholar] [CrossRef]
- LaGrow, A.P.; Ward, M.R.; Lloyd, D.C.; Gai, P.L.; Boyes, E.D. Visualizing the Cu/Cu2O interface transition in nanoparticles with environmental scanning transmission electron microscopy. J. Am. Chem. Soc. 2017, 139, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Goula, M.A.; Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Tsiaoussis, I.; Panagiotopoulou, P.; Goula, G.; Yentekakis, I.V. Syngas production via the biogas dry reforming reaction over Ni supported on zirconia modified with CeO2 or La2O3 catalysts. Int. J. Hydrogen Energ. 2016, 1–17. [Google Scholar] [CrossRef]
- Papageridis, K.N.; Siakavelas, G.; Charisiou, N.D.; Avraam, D.G.; Tzounis, L.; Kousi, K.; Goula, M.A. Comparative study of Ni, Co, Cu supported on γ-alumina catalysts for hydrogen production via the glycerol steam reforming reaction. Fuel Process. Technol. 2016, 152, 156–175. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Avraam, D.G.; Goula, M.A. Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J. Nat. Gas Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]











| Sample | Cu (wt %) | Sn (wt %) | SBET (m2·g−1) | Vpore (cm3·g−1) | Dpore (nm) |
|---|---|---|---|---|---|
| Cu/SiO2 | 10.94 | - | 246.7 | 0.67 | 10.1 |
| Cu-0.3%Sn/SiO2 | 10.82 | 0.29 | 244.9 | 0.65 | 10.2 |
| Cu-0.6%Sn/SiO2 | 10.86 | 0.58 | 244.3 | 0.64 | 10.2 |
| Cu-1.2%Sn/SiO2 | 10.81 | 1.18 | 236.3 | 0.62 | 10.3 |
| Cu-1.8%Sn/SiO2 | 10.79 | 1.82 | 221.8 | 0.59 | 10.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, D.; Dai, B. Promotive Effect of Sn2+ on Cu0/Cu+ Ratio and Stability Evolution of Cu/SiO2 Catalyst in the Hydrogenation of Dimethyl Oxalate. Catalysts 2017, 7, 122. https://doi.org/10.3390/catal7040122
Zhang C, Wang D, Dai B. Promotive Effect of Sn2+ on Cu0/Cu+ Ratio and Stability Evolution of Cu/SiO2 Catalyst in the Hydrogenation of Dimethyl Oxalate. Catalysts. 2017; 7(4):122. https://doi.org/10.3390/catal7040122
Chicago/Turabian StyleZhang, Chuancai, Denghao Wang, and Bin Dai. 2017. "Promotive Effect of Sn2+ on Cu0/Cu+ Ratio and Stability Evolution of Cu/SiO2 Catalyst in the Hydrogenation of Dimethyl Oxalate" Catalysts 7, no. 4: 122. https://doi.org/10.3390/catal7040122
APA StyleZhang, C., Wang, D., & Dai, B. (2017). Promotive Effect of Sn2+ on Cu0/Cu+ Ratio and Stability Evolution of Cu/SiO2 Catalyst in the Hydrogenation of Dimethyl Oxalate. Catalysts, 7(4), 122. https://doi.org/10.3390/catal7040122