1. Introduction
Environmental protection is currently one of the hot topics, globally, so measures for solving air pollution are of significance and represent a great challenge for human beings. To this end, stringent environmental legislation impels the updating of fuel specification to higher levels; in particular, the sulfur content of transportation fuels is to be limited to lower than 10 ppm, and even to near 0 ppm in the near future [
1]. Till now, hydrodesulfurization (HDS) has been the most widely applied technology for removing sulfur from fuels in modern refineries. The main purpose of HDS is to transform poor-quality diesel into an ultraclean final product, while the keypoint is the novel HDS catalyst. However, traditional Al
2O
3 support used in commercial hydrotreating catalysts cannot completely achieve ultra-deep HDS due to its single L acid site, amorphous pore structures, and strong metal-support interaction (MSI) over alumina support [
2,
3]. Alternative methods include improving the acidity and pore structure of the supports by introducing acidic porous materials into the catalyst system. Furthermore, the synergistic effects of B and L acid sites are essential to HDS, hydroisomerization and hydrodearomatization reactions [
2,
3]. Thus, the development and synthesis of novel acidic catalytic materials with low cost will be an important aspect of realizing the production of ultra-clean diesel.
In terms of HDS catalysts, their supports generally require open and interconnected pore structures to eliminate the steric effect of refractory reactants like 4,6-dimethyldibenzothiophene (4,6-DMDBT), and also require a suitable acid property for the desulfurization reactions to take place.
Since the discovery of M41S mesoporous silicas, symbolizing a significant finding in the material synthesis field, mesoporous materials have aroused much more attention in terms of their synthesis, modification and application. However, pure silica has an electrically neutral framework and therefore expresses no acid sites, resulting in a relatively weak acid catalytic reaction [
4]. Recently, many studies have been devoted to the incorporation of Al, Ti, Zr, Sn and V species into the framework of SBA-15 for modulating redox and acidity properties [
5]. Typical methods have included “post synthesis” [
4,
6,
7] and “direct synthesis” [
8,
9] grafting procedures. Direct synthesis methods incorporate aluminum species into the original synthesis system before hydrothermal processing under specialized conditions; however, the respective structures of the materials and the structure order are usually destroyed as the metal content increases. Therefore, some previous studies [
4,
10,
11,
12] have reported that Al could be effectively incorporated into silicas via various post-synthesis processes with anhydrous AlCl
3 [
10], aluminum isopropoxide [
11] or with sodium aluminate [
12] followed by calcination. The incorporated alumina in the tetrahedral framework are associated with the presence of accessible hydroxyl groups in a silica matrix, bringing more acid sites to the molecular sieve, which then influence the catalytic properties of the materials [
4,
13].
Mokaya et al. [
11] proved that Al-containing MCM-41 in good order could be synthesized using a post-synthesis method by grafting Al-alkoxide with siliceous MCM-41 in nonaqueous media. They found that the final materials retained the hexagonal pore structure and characteristics of the parent material MCM-41, and exhibited higher Brönsted acid amounts compared with the counterpart Al-MCM-41 produced by conventional direct-synthesis methods. The authors attributed the higher acid contents to the existence of B acid generation from Al species.
Luan et al. [
4] synthesized SBA-15 material and incorporated it with Al species to obtain Al-SBA-15 via three different post-synthesis routes by reacting SBA-15 with different Al resources, including AlCl
3, aluminum isopropoxide and sodium aluminate. The characterization results showed that the Si/Al ratios in the final products were in accordance with the compositions in the post-synthesis mixtures, indicating the fact that alumina were mostly implanted into the silica SBA-15 materials in a range of Si/Al = 10–40.
Suresh et al. [
14] reported systematic research of sulfided Mo-Ni catalysts over Al-SBA-15 through a post-synthetic method. The catalytic results showed that the improved hydro-denitrogenation activity on the aluminum incorporated catalysts could be mainly ascribed to the substitution of Al, causing the formation of active phases.
The high expense of the surfactants in the synthesis of mesoporous materials impedes wide application in industrial production; meanwhile, the development of low-cost catalysts favors large-scale manufacturing processes [
15]. Based on the above strategy, there has been an increasing taste for the application of “surfactant-free” methods of synthesizing mesoporous materials (such as silica, titania and aluminiumphosphate (ALPOs)) by using small, low-cost organic molecules (e.g., citric acid, urea, tartaric acid or cyclodextrins, among others) [
16,
17,
18,
19,
20].
In 2001, purely siliceous TUD-1 was first published by Jansen et al. [
21]. It was synthesized using a cost-effective synthesis (surfactant-free) method using triethanolamine (TEA) as a template. TUD-1 possesses many advantages compared to other mesoporous materials, such as high surface area, sponge-like interconnecting porous structures, good basement accessibility and tunable pore size distribution [
22,
23], all of which make it a potential support additive for HDS catalysts.
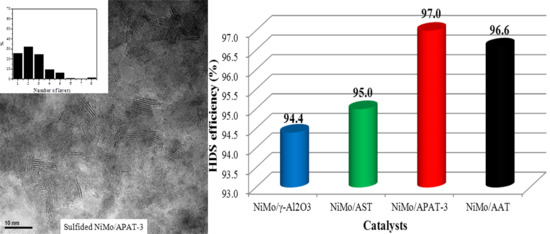
Owing to the good performance of Al incorporated into supports through post-synthetic methods, and the advantages of the material TUD-1, a series of PAT materials with different Al contents produced via a post-synthetic alumination procedure were successfully synthesized by using aluminum isopropoxide in organic solvents as the Al source. Then the as-synthesized materials were adopted as support additives, and mixed with the commercial γ-Al2O3 through a mechanical grinding method to prepare the HDS catalyst for FCC diesel hydrotreating. The supported catalysts were prepared using Ni and Mo as the active metals. The as-synthesized supports and the corresponding NiMo catalysts were analyzed using N2 adsorption-desorption, XRD, SEM, Py-IR, 27Al MAS NMR, UV-vis, H2-TPR ICP and HRTEM methods. The catalytic performances of all catalysts were tested with FCC diesel fuel as feedstock. The correlation between the HDS activities and the physicochemical properties of the catalysts were analyzed systematically. Compared with other catalysts, the catalyst NiMo/APAT-3 had the highest HDS efficiency (97.0%).
3. Discussion
To further investigate the effects of properties on their HDS performances for FCC diesel, systematic research on the correlation of HDS efficiencies with the synergistic effects of textural properties, acidity, MSI and morphology of the sulfided active metal species was discussed.
Generally, catalytic performances are closely concerned with the textural characteristics of catalysts. The addition of mesoporous support additives improves the overall pore structure of the support system of the catalyst. From
Table 6, it can be found that the catalyst of NiMo/APAT-3 has a higher surface area, at around 225.6 m
2 g
−1, and a higher volume, at around 0.40 cm
3 g
−1, than catalysts of NiMo/γ-Al
2O
3 (167.4 m
2 g
−1, 0.34 m
2 g
−1) and NiMo/APAT-4 (224.2 m
2 g
−1, 0.39 m
2 g
−1). As is known to all, high surface area is proper for good distribution of active phases, while large pore diameter facilitates the elimination of diffusion resistance.
Acidity property is another important factor for HDS performance. Although the catalyst NiMo/AST has better textural properties than the series catalysts NiMo/APAT-x, it still has inferior HDS efficiency to that of the latter due to the lower number of acid sites. The incorporation of Al species into the PAT material and the obtained materials used as support additives could modulate the acid property of silica. According to FT-IR results (in
Table 3), the material PAT-3 has the highest total number of weak sites (79.3 µmol g
−1), medium and strong sites (38.6 µmol g
−1); moreover, the highest number of B acid sites. The presence of L acid sites facilitates the hydrogenation reaction; in the meantime, B acid sites favor C-S and C-C bond cleavage [
2], which are very important for the hydrodesulfurization reaction. Moreover, acidity accelerates the dealkylation and isomerization reactions, transforming the refractory sulfur reactants into more reactive components and consequently improving the HDS efficiency [
54]. Thus, the catalyst NiMo/APAT-3 has outstanding HDS activity compared to the remaining catalysts.
Aside from the aforementioned factors, moderate MSI and the morphology of the active phases over the catalysts can also influence the HDS efficiency. The implanting of Al elements into the Si-TUD-1 material adjusts the active metal distributions and modifies the MSI, consistent with H2-TPR analysis. H2-TPR profiles indicate that the catalyst NiMo/APAT-3 has two lower reduction peaks (at 722 and 1069 K), compared to catalyst NiMo/γ-Al2O3 (at 750 and 1142 K), which suggests that the PAT material support additive possesses a lower MSI derived from the siliceous framework of TUD-1, and leads to the generation of Mo species in octahedral coordination, the precursors to forming more “type II” phases with brim and edge sites responsible for hydrodesulfurization.
The strong MSI over the sulfided catalyst NiMo/γ-Al
2O
3 results in the long length (3.9 nm) and low stack number (1.6) of the MoS
2 slabs, facilitating the formation of more “type I” active phases that possess fewer brim and edge active sites, and therefore contribute to a lower activity. The catalyst NiMo/APAT-3 exhibits relatively short lengths (3.2 nm) and suitable layer number (2.5) of the MoS
2 slabs, indicating most active sites existed as “type II”, which are essential for S removal by perpendicular adsorption through the sulfur atom of the reactant molecules [
51,
55]. Moreover, the f
Mo parameter of the catalyst NiMo/APAT-3 (0.31) is significantly higher than that of catalyst NiMo/γ-Al
2O
3 (0.26), confirming that the catalyst NiMo/APAT-3 possesses more active edge Mo atoms than the latter. The catalyst NiMo/AAT with the support additive of Al-TUD-1 has a similar high f
Mo parameter (0.32) to the catalyst NiMo/APAT-3, while having a higher layer number (2.8). The high layer number of the MoS
2 slabs leads to the decrease of the total amount of accessible sites, since only the top layer of multi-stacks can be exposed to the brim sites [
56]. These theoretical explanations are suggested to be the underlying reasons for the HDS activity order: NiMo/APAT-3 > NiMo/AAT > NiMo/γ-Al
2O
3.
The research synthesized Al-modified TUD-1 materials through the post-synthesis method, which possessed appropriate textural properties and suitable acidity, and the PAT material support additive facilitated to the lower MSI in the catalyst. Therefore, NiMo/APAT-3 exhibited the highest HDS efficiency, which would be a promising catalyst for the industrial hydrotreating technique.
4. Materials and Methods
4.1. Materials
Siliceous TUD-1 mesoporous material was produced following the procedure publicized by Jansen et al. [
21] by one-pot sol-gel technique. Typically, a mixture of TEA (≥98%, Sinopharm Chemical Reagent, Co., Ltd (SCRC), shanghai, China) and water was added drop-wise to a certain amount of tetraethyl orthosilicate (TEOS) under vigorous stirring. Subsequently, a controlled number of TEAOH (25 wt %) was dropped into the above mixture. A clear gel was obtained having a molar ratio composition of SiO
2:1TEA:0.2TEAOH:11H
2O. After stirring for some minutes, the final mixture was aged for 24 h, and then dried at 373 K, followed by a hydrothermal treatment at 453 K for 6 h, finally calcined at 873 K for 10 h with a ramp of 274 K/min. After the above steps, purely Siliceous TUD-1 was obtained.
The siliceous TUD-1 was adopted as a parent material to prepare Al incorporation TUD-1 by post-synthesis method [
37]. Various amounts of aluminum isopropoxide (≥98%, SCRC) were initially dissolved in isopropanol, then the obtained Siliceous TUD-1 was added to the above mixture under stirring for 3–5 h. After suction filtration and washing with dry isopropoxide, the solid compound was finally calcined at 823 K for 6 h. For the sake of expression, the alkoxide addition amounts were represented by Al
2O
3 percentage, then the synthesized materials were named as PAT-x, where PAT represents post-synthesis aluminum modified TUD-1 and x represents the weight ratio of Al
2O
3, x = 1, 2, 3 and 4, corresponding to Al
2O
3 percentages of 3%, 6%, 12% and 18%, respectively.
Aluminum in-situ modified TUD-1 noted as Al-TUD-1 was synthesized via the conventional direct synthesis method described in the literature [
38] with a Si/Al value of 30.
4.2. Production of Supports and Catalysts
The supports of each catalyst were made up of 70 wt % commercial γ-Al2O3 and 30 wt % different support additives such as Siliceous TUD-1, Al-TUD-1 or PAT-x material through the mechanical grinding method. The corresponding catalysts were produced via a two-step incipient impregnation technique using ammonium heptamolybdate as Mo source and nickel nitrate as Ni source. After each step, the as-prepared samples were dispersed by a sonic bath for 30 min. The final samples were calcined at 823 K for 4 h after dried at 383 K for 4 h.
All catalysts have the same active metal contents (MoO3 15.5 wt % and NiO 3.5 wt %) and support proportion (81 wt %).
Furthermore, the catalysts were designated as NiMo/γ-Al2O3-Si-TUD-1 (NiMo/AST), NiMo/γ-Al2O3-Al-TUD-1 (NiMo/AAT), NiMo/γ-Al2O3-PAT-1 (NiMo/APAT-1), NiMo/γ-Al2O3-PAT-2 (NiMo/APAT-2), NiMo/γ-Al2O3-PAT-3 (NiMo/APAT-3) and NiMo/γ-Al2O3-PAT-4 (NiMo/APAT-4). The traditional NiMo/γ-Al2O3 catalyst was used as the reference.
4.3. Characterization of Supports and Catalysts
The supports and catalysts were analyzed using N2 adsorption-desorption, XRD, ICP, SEM, Py-IR, 27Al MAS NMR, UV-vis, H2-TPR and HRTEM.
N2 adsorption-desorption were performed at 77 K on instrument of a Micromeritics TriStar II 2020 porosimetry analyzer. Prior to each measurement, about 0.2 g samples were degassed at 623 K for 4 h under high vacuum. The surface area was via the BET method. The pore size was via the BJH method.
XRD patterns were measured on an instrument of Shimadzu X-6000 using Cu Kα radiation operated at a ray tube voltage of 40 kV, small angle range: 2θ of 0.7°~10°, bighorn range: 2θ of 10°~90°.
The SEM images of the catalysts were characterized on a Quanta 200 F instruments, combined with a Tecnai F20 electron microscopy for the EDS elemental mapping technology.
27Al MAS NMR were analyzed on a Bruker MSL-300NMR spectrometer.
The UV-vis diffuse reflectance spectra were measured on a Hitachi U-4100 spectrophotomer equipped with an integration sphere diffuse reflectance attachment.
The Py-IR experiments were operated on a MAGNAIR 560 FTIR instrument.
H2-TPR analysis was tested using a Quantachrome apparatus (Autosorb-iQ, USA).
HRTEM of the supported catalysts were characterized on a Philips Tecnai G2 F20 transmission electron.
4.4. Catalytic Activity Measurement
The HDS activity were evaluated in a fixed bed reactor using FCC diesel as feedstock from PetroChina Hohhot petrochemical company, with S content and N content are 1013.8 mg/L and 640.3 mg/L respectively.
Prior to the test of catalytic performance, 2 g catalyst with a diameter of 40–60 mesh was presulfided by 2 wt % CS2-cyclohexane and H2/cyclohexane ratio of 600 for 4 h under the conditions of 593 K and 4 MPa.
After presulfidation, the HDS performances were evaluated at the temperature of 623 K, meanwhile the pressure of 5.0 MPa, H2 to Oil ratio of 600 and weight hourly space velocity (WHSV) of 1.0 h−1. The final product sample was collected at steady state after 9 h on stream, and each evaluation experiment has been made three times in parallel, and taken the average to calculate the catalytic hydrodesulfurization efficiency. The S amounts were obtained by using a RPP-2000SN sulfur detector. The measurement deviation is within 2 mg/L. The final HDS efficiency of each catalyst is the average value of these 3 samples.
The HDS efficiency is obtained by the Equation (3).
where S
f refers to the amount of sulfur in feed, and S
p refers to the sulfur in product.
5. Conclusions
Siliceous TUD-1 mesoporous material was prepared by one-pot sol-gel technique. Then PAT materials with different Al contents adopting aluminum isopropoxide to be the Al sources in organic solvents were prepared through the post-synthesis method. The as-synthesized supports and the corresponding NiMo catalysts were analyzed using XRD, N2-adsorption, SEM, Py-IR, 27Al MAS NMR, UV-vis, H2-TPR ICP and HRTEM methods. The characterizations of XRD and N2-adsorption showed that the post-synthesis Al grafting process had no significant influence on the pore structure of Si-TUD-1. Furthermore, the results of Py-IR and 27Al MAS NMR indicated that the addition of Al species could bring Lewis and B acid sites into Si-TUD-1 material, and the material of PAT-3 with suitable amounts of Al content had the highest total acidity sites and B acid sites. The supports of each catalyst were the mechanical mixture of the series PAT materials and the commercial γ-Al2O3. The corresponding catalysts were prepared with Ni and Mo as the active metals. The catalysts of NiMo/γ-Al2O3 and NiMo/AAT were prepared as well, and were used as the reference. The H2-TPR results showed that the addition of PAT materials into the support system could result in the lower reduction peaks, and led to the generation of octahedral Mo elements with the easier redox and presulfurization properties. The HRTEM analysis exhibited that the sulfided catalyst NiMo/APAT-3 had a relatively short length (3.2 nm) and suitable stack (2.5) of the MoS2 slabs.
The HDS efficiencies of all the supported catalysts were also evaluated with FCC diesel as feedstock. The catalytic results confirmed that the catalyst NiMo/APAT-3 possessed the highest HDS efficiency (97.0%), which could be associated with the synergistic effects of the appreciate textural characteristics, higher acidity, moderate MSI, relatively short length and suitable stacking of the MoS2 slabs.