Co3O4 Nanoparticle-Decorated N-Doped Mesoporous Carbon Nanofibers as an Efficient Catalyst for Oxygen Reduction Reaction
Abstract
:1. Introduction
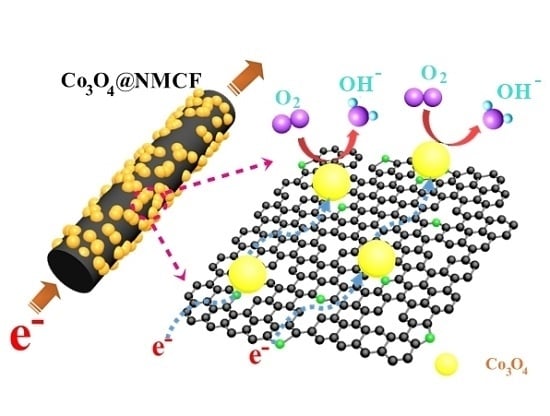
2. Results and Discussion
3. Materials and Methods
3.1. Fabrication of Co3O4 NP-Decorated N-Doped Mesoporous Carbon Nanofibers
3.2. Material Characterizations
3.3. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Porter, N.; Wu, H.; Quan, Z.; Fang, J. Shape-controlled and electrocatalytic activity-enhancement of Pt-based bimetallic nanocrystals. Acc. Chem. Res. 2013, 46, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yamauchi, Y. Metallic Nanocages: Synthesis of bimetallic Pt–Pd hollow nanoparticles with dendritic shells by selective chemical etching. J. Am. Chem. Soc. 2013, 135, 16762–16765. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, H.; Markovic, N. Just a dream—Or future reality? Science 2009, 324, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Debe, M. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Bashyam, R.; Zelenay, P. A class of non-precious metal composite catalysts for fuel cells. Nature 2006, 443, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Proietti, E.; Jaouen, F.; Dodelet, J. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, H.; Zhou, J.; Li, Y.; Wang, J.; Regier, T.; Dai, H. Covalent hybrid of spinel manganese—Cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2012, 134, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Kuai, L.; Geng, B. A template-free route to a Fe3O4-Co3O4 yolk-shell nanostructure as a noble-metal free electrocatalyst for ORR in alkaline media. J. Mater. Chem. 2012, 22, 19132–19138. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Dai, H. Strongly coupled inorganic/nanocarbon hybrid materials for advanced electrocatalysis. J. Am. Chem. Soc. 2013, 135, 2013–2036. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Wei, Z.H.; Huang, T.; You, Y.; Chen, J. High-performance bi-functional electrocatalysts of 3D crumpled grapheme-cobalt oxide nanohybrids for oxygen reduction and evolution reactions. Energy Environ. Sci. 2014, 7, 609–616. [Google Scholar] [CrossRef]
- Xue, H.; Wu, S.; Tang, J.; Gong, H.; He, P.; He, J.; Zhou, H. Hierarchical Porous Nickel Cobaltate Nanoneedle Arrays as Flexible Carbon-Protected Cathodes for High-Performance Lithium–Oxygen Batteries. ACS Appl. Mater. Interfaces 2016, 8, 8427–8435. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Xue, H.; Wang, T.; Guo, H.; Fan, X.; Song, L.; Xia, W.; He, J. High-loading nickel cobaltate nanoparticles anchored on three-dimensional n-doped graphene as an efficient bifunctional catalyst for lithium-oxygen batteries. ACS Appl. Mater. Interfaces 2016, 8, 18060–18068. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, H.; Diao, P.; Chang, W.; Hong, G.; Li, Y.; Gong, M.; Xie, L.; Zhou, J.; Wang, J.; et al. Oxygen reduction electrocatalyst based on strongly coupled cobalt oxide nanocrystals and carbon nanotubes. J. Am. Chem. Soc. 2012, 134, 15849–15857. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Zhang, J.; Xie, Z.; Wang, X.; Lin, T. Preparation, structure and supercapacitance of bonded carbon nanofiber electrode materials. Carbon 2011, 49, 2380–2388. [Google Scholar] [CrossRef]
- Gong, K.; Chakrabarti, S.; Dai, L. Electrochemistry at carbon nanotube electrodes: Is the nanotube tip more active than the sidewall? Angew. Chem. Int. Ed. 2008, 47, 5446–5450. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Mu, X.; Tang, J.; Fan, X.; Gong, H.; Wang, T.; He, J.; Yamauchi, Y. A nickel cobaltate nanoparticle-decorated hierarchical porous N-doped carbon nanofiber film as a binder-free self-supported cathode for nonaqueous Li–O2 batteries. J. Mater. Chem. A 2016, 4, 9106–9112. [Google Scholar] [CrossRef]
- Rats, D.; Vandenbulcke, L.; Herbin, R.; Benoit, R.; Erre, R.; Serin, V.; Sevely, J. Characterization of diamond films deposited on titanium and its alloys. Thin Solid Films 1995, 270, 177–183. [Google Scholar] [CrossRef]
- Coutures, J.; Erre, R.; Massiot, D.; Landron, C.; Billard, D.; Peraudeau, G. Ar+ ion beam effects on MxOy-alumina silica glasses. Radiat. Eff. 1986, 98, 83–91. [Google Scholar] [CrossRef]
- Xue, H.; Zhao, J.; Tang, J.; Gong, H.; He, P.; Zhou, H.; Yamauchi, Y.; He, J. High-loading nano-SnO2 encapsulated in situ in three-dimensional rigid porous carbon for superior lithium-ion batteries. Chem. Eur. J. 2016, 22, 4915–4923. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Wang, T.; Zhao, J.; Gong, H.; Tang, J.; Guo, H.; Fan, X.; He, J. Constructing a multicomponent ordered mesoporous carbon for improved electrochemical performance induced by in-situ doping phosphorus. Carbon 2016, 104, 10–19. [Google Scholar] [CrossRef]
- Cui, B.; Lin, H.; Liu, Y.; Li, J.; Sun, P.; Zhao, X.; Liu, C. Photophysical and photocatalytic properties of core-ring structured NiCo2O4 nanoplatelets. J. Phys. Chem. C 2009, 113, 14083–14087. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Hou, L.; Zhang, X.; Shen, L.; Lou, X. Ultrathin mesoporous NiCo2O4 nanosheets supported on ni foam as advanced electrodes for supercapacitors. Adv. Funct. Mater. 2012, 22, 4592–4597. [Google Scholar] [CrossRef]
- Tang, J.; Wang, T.; Pan, X.; Sun, X.; Fan, X.; Guo, Y.; Xue, H.; He, J. Synthesis and Electrochemical Characterization of N-Doped Partially Graphitized Ordered Mesoporous Carbon–Co Composite. J. Phys. Chem. C 2013, 3, 16896–16906. [Google Scholar] [CrossRef]
- Tang, J.; Liu, J.; Li, C.; Li, Y.; Tade, M.; Dai, S.; Yamauchi, Y. Synthesis of Nitrogen-Doped Mesoporous Carbon Spheres with Extra-Large Pores through Assembly of Diblock Copolymer Micelles. Angew. Chem. Int. Ed. 2015, 54, 588–593. [Google Scholar] [CrossRef]
- Xue, H.; Tang, J.; Gong, H.; Guo, H.; Fan, X.; Wang, T.; He, J.; Yamauchi, Y. Fabrication of PdCo bimetallic nanoparticles anchored on three-dimensional ordered n-doped porous carbon as an efficient catalyst for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2016, 8, 20766–20771. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, T.; Ma, Y.; Xue, H.; Guo, H.; Fan, X.; Xia, W.; Gong, H.; He, J. Functional species encapsulated in nitrogen-doped porous carbon as a highly efficient catalyst for the oxygen reduction reaction. Chem. Eur. J. 2017, 23, 3398–3405. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, T.; Wang, Y.; Xue, H.; Fan, X.; Guo, H.; Xia, W.; Gong, H.; He, J. Porous iron-tungsten carbide electrocatalyst with high activity and stability toward oxygen reduction reaction: From the self-assisted synthetic mechanism to its active-species probing. ACS Appl. Mater. Interfaces 2017, 9, 3713–3722. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, H.; Wang, T.; Gong, H.; Guo, H.; Fan, X.; Song, L.; Xia, W.; Feng, Y.; He, J. Co3O4 Nanoparticle-Decorated N-Doped Mesoporous Carbon Nanofibers as an Efficient Catalyst for Oxygen Reduction Reaction. Catalysts 2017, 7, 189. https://doi.org/10.3390/catal7060189
Xue H, Wang T, Gong H, Guo H, Fan X, Song L, Xia W, Feng Y, He J. Co3O4 Nanoparticle-Decorated N-Doped Mesoporous Carbon Nanofibers as an Efficient Catalyst for Oxygen Reduction Reaction. Catalysts. 2017; 7(6):189. https://doi.org/10.3390/catal7060189
Chicago/Turabian StyleXue, Hairong, Tao Wang, Hao Gong, Hu Guo, Xiaoli Fan, Li Song, Wei Xia, Yaya Feng, and Jianping He. 2017. "Co3O4 Nanoparticle-Decorated N-Doped Mesoporous Carbon Nanofibers as an Efficient Catalyst for Oxygen Reduction Reaction" Catalysts 7, no. 6: 189. https://doi.org/10.3390/catal7060189
APA StyleXue, H., Wang, T., Gong, H., Guo, H., Fan, X., Song, L., Xia, W., Feng, Y., & He, J. (2017). Co3O4 Nanoparticle-Decorated N-Doped Mesoporous Carbon Nanofibers as an Efficient Catalyst for Oxygen Reduction Reaction. Catalysts, 7(6), 189. https://doi.org/10.3390/catal7060189