Fast and Large-Scale Anodizing Synthesis of Pine-Cone TiO2 for Solar-Driven Photocatalysis
Abstract
:1. Introduction
2. Results and Discussion
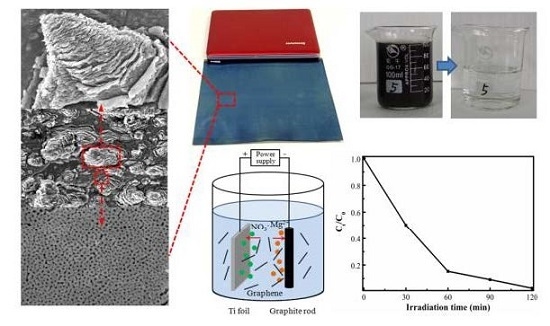
2.1. Morphology
2.2. Proposed Formation Mechanism
2.3. Crystal Phase and Surface Chemical Composition
2.4. Lattice Defects and Optical Absorption
2.5. Photoelectrochemical Activity
2.6. Photoelectrocatalytic Activity
2.7. Photoelectrocatalytic Degradation of Wastewater
3. Experimental Section
3.1. Fast Anodization Synthesis of the TiO2 Nanostructured Film
3.2. Fast Anodization Synthesis of Large-Scale TiO2 Film
3.3. Characterization
3.4. Photoelectrochemical Performance
3.5. Photoelectrocatalytic Activity in Hydrogen Generation and Removal of Model Organic Pollutants
3.6. Removal of Organic Pollutants in Wastewater
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dong, J.; Han, J.; Liu, Y.; Nakajima, A.; Matsushita, S.; Wei, S.; Gao, W. Defective Black TiO2 Synthesized via Anodization for Visible-Light Photocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhao, W.; Yang, C.; Yin, H.; Lin, T.; Shan, Y.; Xie, Y.; Gu, H.; Huang, F. Black TiO2 nanotube arrays for high-efficiency photoelectrochemical water-splitting. J. Mater. Chem. A 2014, 2, 8612–8616. [Google Scholar] [CrossRef]
- Su, T.; Yang, Y.; Na, Y.; Fan, R.; Li, L.; Wei, L.; Yang, B.; Cao, W. An Insight into the Role of Oxygen Vacancy in Hydrogenated TiO2 Nanocrystals in the Performance of Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 3754–3763. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hwang, S.H.; Yun, J.; Jang, J. Fabrication of SiO2/TiO2 Double-Shelled Hollow Nanospheres with Controllable Size via Sol-Gel Reaction and Sonication-Mediated Etching. ACS Appl. Mater. Interfaces 2014, 6, 15420–15426. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Jeon, N.J.; Noh, J.H.; Seok, S.I. Well-Organized Mesoporous TiO2 Photoelectrodes by Block Copolymer-Induced Sol-Gel Assembly for Inorganic-Organic Hybrid Perovskite Solar Cells. J. Phys. Chem. C 2014, 118, 16688–16693. [Google Scholar] [CrossRef]
- Lee, J.S.; You, K.H.; Park, C.B. Highly photoactive, low bandgap TiO2 nanoparticles wrapped by graphene. Adv. Mater. 2012, 24, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J. Hydrothermal Synthesis of Graphene-TiO2 Nanotube Composites with Enhanced Photocatalytic Activity. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Chen, M.; Shen, X.; Wu, Q.; Li, W.; Diao, G. Template-assisted synthesis of core-shell α-Fe2O3@TiO2 nanorods and their photocatalytic property. J. Mater. Sci. 2015, 50, 4083–4094. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Huang, X.; Huang, C.; Li, H. Synthesis and characterization of polypyrrole using TiO2 nanotube@poly(sodium styrene sulfonate) as dopant and template. Polym. Compos. 2016, 37, 462–467. [Google Scholar] [CrossRef]
- Dutta, S.; Patra, A.K.; De, S.; Bhaumik, A.; Saha, B. Self-Assembled TiO2 Nanospheres By Using a Biopolymer as a Template and Its Optoelectronic Application. ACS Appl. Mater. Interfaces 2012, 4, 1560–1564. [Google Scholar] [CrossRef] [PubMed]
- Zimbone, M.; Cacciato, G.; Boutinguiza, M.; Privitera, V.; Grimaldi, M.G. Laser irradiation in water for the novel, scalable synthesis of black TiOx photocatalyst for environmental remediation. Beilstein J. Nanotechnol. 2017, 8, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Zimbone, M.; Cacciato, G.; Sanz, R.; Carles, R.; Gulino, A.; Privitera, V.; Grimaldi, M.G. Black TiOx photocatalyst obtained by laser irradiation in water. Catal. Commun. 2016, 84, 11–15. [Google Scholar] [CrossRef]
- Zimbone, M.; Cacciato, G.; Buccheri, M.A.; Sanz, R.; Piluso, N.; Reitano, R.; Via, F.L.; Grimaldi, M.G.; Privitera, V. Photocatalytical activity of amorphous hydrogenated TiO2 obtained by pulsed laser ablation in liquid. Mater. Sci. Semicond. Process. 2016, 42, 28–31. [Google Scholar] [CrossRef]
- Zimbone, M.; Buccheri, M.A.; Cacciato, G.; Sanz, R.; Rappazzo, G.; Boninelli, S.; Reitano, R.; Romano, L.; Privitera, V.; Grimaldi, M.G. Photocatalytical and antibacterial activity of TiO2 nanoparticles obtained by laser ablation in water. Appl. Catal. B Environ. 2015, 165, 487–494. [Google Scholar] [CrossRef]
- Du, Y.; Cai, H.; Wen, H.; Wu, Y.; Huang, L.; Ni, J.; Li, J.; Zhang, J. Novel Combination of Efficient Perovskite Solar Cells with Low Temperature Processed Compact TiO2 Layer via Anodic Oxidation. ACS Appl. Mater. Interfaces 2016, 8, 12836–12842. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, J.-W.; Shu, S.; Zhang, J.; Zheng, L.; Tsang, C.K.; Cheng, H.; Liang, F.; Lee, S.-T.; Li, Y.Y. Selective Removal of the Outer Shells of Anodic TiO2 Nanotubes. Small 2013, 9, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sun, C.; Yang, H.G.; Smith, S.C.; Wang, L.; Lu, G.Q.; Cheng, H.-M. Nanosized anatase TiO2 single crystals for enhanced photocatalytic activity. Chem. Commun. 2010, 46, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Wang, L.; Liu, G.; Lu, G.Q.; Cheng, H.-M. Hollow Anatase TiO2 Single Crystals and Mesocrystals with Dominant {101} Facets for Improved Photocatalysis Activity and Tuned Reaction Preference. ACS Catal. 2012, 2, 1854–1859. [Google Scholar] [CrossRef]
- Wu, X.; Lu, G.Q.; Wang, L. Shell-in-shell TiO2 hollow spheres synthesized by one-pot hydrothermal method for dye-sensitized solar cell application. Energy Environ. Sci. 2011, 4, 3565–3572. [Google Scholar] [CrossRef]
- Joo, J.B.; Dahl, M.; Li, N.; Zaera, F.; Yin, Y. Tailored synthesis of mesoporous TiO2 hollow nanostructures for catalytic applications. Energy Environ. Sci. 2013, 6, 2082–2092. [Google Scholar] [CrossRef]
- Joo, J.B.; Zhang, Q.; Dahl, M.; Lee, I.; Goebl, J.; Zaera, F.; Yin, Y. Control of the nanoscale crystallinity in mesoporous TiO2 shells for enhanced photocatalytic activity. Energy Environ. Sci. 2012, 5, 6321–6327. [Google Scholar] [CrossRef]
- Konishi, J.; Fujita, K.; Nakanishi, K.; Hirao, K. Monolithic TiO2 with Controlled Multiscale Porosity via a Template-Free Sol-Gel Process Accompanied by Phase Separation. Chem. Mater. 2006, 18, 6069–6074. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Z.; Lu, G.Q.; Wang, L. Nanosized Anatase TiO2 Single Crystals with Tunable Exposed (001) Facets for Enhanced Energy Conversion Efficiency of Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2011, 21, 4167–4172. [Google Scholar] [CrossRef]
- Sánchez-Tovar, R.; Lee, K.; García-Antón, J.; Schmuki, P. Formation of anodic TiO2 nanotube or nanosponge morphology determined by the electrolyte hydrodynamic conditions. Electrochem. Commun. 2013, 26, 1–4. [Google Scholar] [CrossRef]
- Yoo, J.E.; Lee, K.; Altomare, M.; Selli, E.; Schmuki, P. Self-Organized Arrays of Single-Metal Catalyst Particles in TiO2 Cavities: A Highly Efficient Photocatalytic System. Angew. Chem. Int. Ed. 2013, 52, 7514–7517. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Altomare, M.; Mokhtar, M.; Alshehri, A.; Al-Thabaiti, S.A.; Mazare, A.; Schmuki, P. Photocatalytic H2 Generation Using Dewetted Pt-Decorated TiO2 Nanotubes: Optimized Dewetting and Oxide Crystallization by a Multiple Annealing Process. J. Phys. Chem. C 2016, 120, 15884–15892. [Google Scholar] [CrossRef]
- Liang, F.; Luo, L.-B.; Tsang, C.-K.; Zheng, L.; Cheng, H.; Li, Y.Y. TiO2 nanotube-based field effect transistors and their application as humidity sensors. Mater. Res. Bull. 2012, 47, 54–58. [Google Scholar] [CrossRef]
- Zheng, L.; Han, S.; Liu, H.; Yu, P.; Fang, X. Hierarchical MoS2 Nanosheet@ TiO2 Nanotube Array Composites with Enhanced Photocatalytic and Photocurrent Performances. Small 2016, 12, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Inoue, S.; Wada, K.; Hishita, S.; Kurashima, K. A New Electrochemical Lithography Fabrication of Self-Organized Titania Nanostructures on Glass by Combined Anodization. J. Electrochem. Soc. 2005, 152, B116–B124. [Google Scholar] [CrossRef]
- Ghicov, A.; Schmuki, P. Self-ordering electrochemistry: A review on growth and functionality of TiO2 nanotubes and other self-aligned MOx structures. Chem. Commun. 2009, 2791–2808. [Google Scholar] [CrossRef] [PubMed]
- Giammar, D.E.; Maus, C.J.; Xie, L. Effects of particle size and crystalline phase on lead adsorption to titanium dioxide nanoparticles. Environ. Eng. Sci. 2007, 24, 85–95. [Google Scholar] [CrossRef]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Ji, Z. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-H.; Liu, W.-S.; Su, Y.-F. Aggregation of stabilized TiO2 nanoparticle suspensions in the presence of inorganic ions. Environ. Toxicol. Chem. 2012, 31, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Deskins, N.A.; Kerisit, S.; Rosso, K.M.; Dupuis, M. Molecular Dynamics Characterization of Rutile-Anatase Interfaces. J. Phys. Chem. C 2007, 111, 9290–9298. [Google Scholar] [CrossRef]
- Liang, H.C.; Li, X.Z. Effects of structure of anodic TiO2 nanotube arrays on photocatalytic activity for the degradation of 2,3-dichlorophenol in aqueous solution. J. Hazard. Mater. 2009, 162, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, G.; Sato, T.; Kanamori, K.; Nakano, K.; Yajima, T.; Kobayashi, Y.; Kageyama, H.; Abe, T.; Nakanishi, K. Hierarchically Porous Monoliths Based on N-Doped Reduced Titanium Oxides and Their Electric and Electrochemical Properties. Chem. Mater. 2013, 25, 3504–3512. [Google Scholar] [CrossRef]
- Wang, D.-H.; Jia, L.; Wu, X.-L.; Lu, L.-Q.; Xu, A.-W. One-step hydrothermal synthesis of N-doped TiO2/C nanocomposites with high visible light photocatalytic activity. Nanoscale 2012, 4, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mu, K.; Zhong, J.; Chen, K.; Zhang, Y.; Yang, G.; Wang, L.; Deng, S.; Shen, F.; Zhang, X. Design of a solar-driven TiO2 nanofilm on Ti foil by self-structure modifications. RSC Adv. 2015, 5, 41437–41444. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Knözinger, H. Species formed after NO adsorption and NO+ O2 co-adsorption on TiO2: An FTIR spectroscopic study. Phys. Chem. Chem. Phys. 2000, 2, 2803–2806. [Google Scholar] [CrossRef]
- Yang, J.; Bai, H.; Tan, X.; Lian, J. IR and XPS investigation of visible-light photocatalysis-Nitrogen-carbon-doped TiO2 film. Appl. Surf. Sci. 2006, 253, 1988–1994. [Google Scholar] [CrossRef]
- Wang, J.; Tafen, D.N.; Lewis, J.P.; Hong, Z.; Manivannan, A.; Zhi, M.; Li, M.; Wu, N. Origin of photocatalytic activity of nitrogen-doped TiO2 nanobelts. J. Am. Chem. Soc. 2009, 131, 12290–12297. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Qin, Y.; Chen, C.; Guo, X.; Tan, B.; Cai, W. Origin of Visible Light Photoactivity of Reduced Graphene Oxide/TiO2 by in Situ Hydrothermal Growth of Undergrown TiO2 with Graphene Oxide. J. Phys. Chem. C 2013, 117, 16734–16741. [Google Scholar] [CrossRef]
- Zhuang, J.; Weng, S.; Dai, W.; Liu, P.; Liu, Q. Effects of Interface Defects on Charge Transfer and Photoinduced Properties of TiO2 Bilayer Films. J. Phys. Chem. C 2012, 116, 25354–25361. [Google Scholar] [CrossRef]
- Batzill, M.; Morales, E.H.; Diebold, U. Influence of nitrogen doping on the defect formation and surface properties of TiO2 rutile and anatase. Phys. Rev. Lett. 2006, 96, 026103. [Google Scholar] [CrossRef] [PubMed]
- Di Valentin, C.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Giamello, E. Characterization of paramagnetic species in N-doped TiO2 powders by EPR spectroscopy and DFT calculations. J. Phys. Chem. B 2005, 109, 11414–11419. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Luo, Z.; Huang, K.; Lagoudas, D.C. Effect of heat treatment on morphology, crystalline structure and photocatalysis properties of TiO2 nanotubes on Ti substrate and freestanding membrane. Appl. Surf. Sci. 2011, 257, 6451–6461. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of Nature and Location of Defects on Bandgap Narrowing in Black TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Li, N.; Zhang, Y.; Kruger, M.B.; Murowchick, J.; Selloni, A.; Chen, X. Directional Heat Dissipation across the Interface in Anatase-Rutile Nanocomposites. ACS Appl. Mater. Interfaces 2013, 5, 9883–9890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Hedhili, M.N.; Zhang, H.; Wang, P. Plasmonic Gold Nanocrystals Coupled with Photonic Crystal Seamlessly on TiO2 Nanotube Photoelectrodes for Efficient Visible Light Photoelectrochemical Water Splitting. Nano Lett. 2013, 13, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, P. Optimization of photoelectrochemical water splitting performance on hierarchical TiO2 nanotube arrays. Energy Environ. Sci. 2012, 5, 6506–6512. [Google Scholar] [CrossRef]
- Xiao, F.-X.; Hung, S.-F.; Miao, J.; Wang, H.-Y.; Yang, H.; Liu, B. Metal-Cluster-Decorated TiO2 Nanotube Arrays: A Composite Heterostructure toward Versatile Photocatalytic and Photoelectrochemical Applications. Small 2015, 11, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yan, K.; Wang, G.; Guo, W.; Ma, T. Effect of Annealing Temperature on the Hydrogen Production of TiO2 Nanotube Arrays in a Two-Compartment Photoelectrochemical Cell. J. Phys. Chem. C 2011, 115, 12844–12849. [Google Scholar] [CrossRef]
- Ryu, J.; Choi, W. Substrate-Specific Photocatalytic Activities of TiO2 and Multiactivity Test for Water Treatment Application. Environ. Sci. Technol. 2008, 42, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Mcfarlane, M. Current and possible future methods of assessing the activities of photocatalyst films. Catal. Today 2007, 129, 22–28. [Google Scholar] [CrossRef]
- Mills, A.; Hill, C.; Robertson, P.K.J. Overview of the current ISO tests for photocatalytic materials. J. Photochem. Photobiol. A Chem. 2012, 237, 7–23. [Google Scholar] [CrossRef]












| Light Sources | Irradiation Time (min) | Degradation Rate (%) |
|---|---|---|
| UV | 20 | 94 |
| sunlight | 120 | 85 |
| fluorescent lamp | 180 | 16 |
| Initial Concentration (mg L−1) | Final Concentration (mg L−1) | Removal Rate (%) | |
|---|---|---|---|
| COD | 2620 | 253 | 90 |
| TOC | 1030 | 280 | 72.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, Y.; Wang, L.; Yang, G.; Shen, F.; Deng, S.; Zhang, X.; He, Y.; Hu, Y.; Chen, X. Fast and Large-Scale Anodizing Synthesis of Pine-Cone TiO2 for Solar-Driven Photocatalysis. Catalysts 2017, 7, 229. https://doi.org/10.3390/catal7080229
Liu Y, Zhang Y, Wang L, Yang G, Shen F, Deng S, Zhang X, He Y, Hu Y, Chen X. Fast and Large-Scale Anodizing Synthesis of Pine-Cone TiO2 for Solar-Driven Photocatalysis. Catalysts. 2017; 7(8):229. https://doi.org/10.3390/catal7080229
Chicago/Turabian StyleLiu, Yan, Yanzong Zhang, Lilin Wang, Gang Yang, Fei Shen, Shihuai Deng, Xiaohong Zhang, Yan He, Yaodong Hu, and Xiaobo Chen. 2017. "Fast and Large-Scale Anodizing Synthesis of Pine-Cone TiO2 for Solar-Driven Photocatalysis" Catalysts 7, no. 8: 229. https://doi.org/10.3390/catal7080229
APA StyleLiu, Y., Zhang, Y., Wang, L., Yang, G., Shen, F., Deng, S., Zhang, X., He, Y., Hu, Y., & Chen, X. (2017). Fast and Large-Scale Anodizing Synthesis of Pine-Cone TiO2 for Solar-Driven Photocatalysis. Catalysts, 7(8), 229. https://doi.org/10.3390/catal7080229