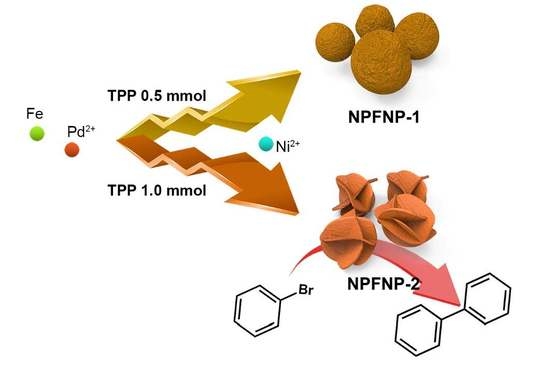
Fabrication of Crumpled Ball-Like Nickel Doped Palladium-Iron Oxide Hybrid Nanoparticles with Controlled Morphology as Effective Catalyst for Suzuki–Miyaura Coupling Reaction
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Typical Synthetic Method of Ni Doped Pd-Fe3O4 Hybrid Nanoparticles (NPFNPs)
3.3. General Procedure for Suzuki–Miyaura Coupling Reactions
3.4. Catalyst Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xiao, C.; Wang, L.L.; Maligal-Ganesh, R.V.; Smetana, V.; Walen, H.; Thiel, P.A.; Miller, G.J.; Johnson, D.D.; Huang, W. Intermetallic NaAu2 as a heterogeneous catalyst for low-temperature co oxidation. J. Am. Chem. Soc. 2013, 135, 9592–9595. [Google Scholar] [CrossRef] [PubMed]
- Maligal-Ganesh, R.V.; Xiao, C.X.; Goh, T.W.; Wang, L.L.; Gustafson, J.; Pei, Y.C.; Qi, Z.Y.; Johnson, D.D.; Zhang, S.R.; Tao, F.; et al. A ship-in-a-bottle strategy to synthesize encapsulated intermetallic nanoparticle catalysts: Exemplified for furfural hydrogenation. ACS Catal. 2016, 6, 1754–1763. [Google Scholar] [CrossRef]
- Jbir, I.; Couble, J.; Khaddar-Zine, S.; Ksibi, Z.; Meunier, F.; Bianchi, D. Individual heat of adsorption of adsorbed co species on palladium and Pd-Sn nanoparticles supported on Al2O3 by using temperature-programmed adsorption equilibrium methods. ACS Catal. 2016, 6, 2545–2558. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Y.W.; Tao, F.F. Shape control of bimetallic nanocatalysts through well-designed colloidal chemistry approaches. Chem. Soc. Rev. 2012, 41, 8050–8065. [Google Scholar] [CrossRef] [PubMed]
- Serov, A.; Kwak, C. Review of non-platinum anode catalysts for DMFC and PEMFC application. Appl. Catal. B Environ. 2009, 90, 313–320. [Google Scholar] [CrossRef]
- Chen, J.; Wiley, B.; McLellan, J.; Xiong, Y.; Li, Z.Y.; Xia, Y. Optical properties of Pd-Ag and Pt-Ag nanoboxes synthesized via galvanic replacement reactions. Nano Lett. 2005, 5, 2058–2062. [Google Scholar] [CrossRef] [PubMed]
- Kuttiyiel, K.A.; Sasaki, K.; Choi, Y.M.; Su, D.; Liu, P.; Adzic, R.R. Nitride stabilized PtNi core-shell nanocatalyst for high oxygen reduction activity. Nano Lett. 2012, 12, 6266–6271. [Google Scholar] [CrossRef] [PubMed]
- Coutts, M.J.; Zareie, H.M.; Cortie, M.B.; Phillips, M.R.; Wuhrer, R.; McDonagh, A.M. Exploiting zinc oxide re-emission to fabricate periodic arrays. ACS Appl. Mater. Interfaces 2010, 2, 1774–1779. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Murugan, A.V.; Manthiram, A. Pt-encapsulated Pd-Co nanoalloy electrocatalysts for oxygen reduction reaction in fuel cells. Langmuir 2010, 26, 2894–2903. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics. Angew. Chem. Int. Ed. Engl. 2009, 48, 60–103. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.J.; Xia, Y.N. Shape-controlled synthesis of metal nanostructures: The case of palladium. Adv. Mater. 2007, 19, 3385–3391. [Google Scholar] [CrossRef]
- Sun, Y.G.; Mayers, B.; Herricks, T.; Xia, Y.N. Polyol synthesis of uniform silver nanowires: A plausible growth mechanism and the supporting evidence. Nano Lett. 2003, 3, 955–960. [Google Scholar] [CrossRef]
- Ha, T.H.; Koo, H.J.; Chung, B.H. Shape-controlled syntheses of gold nanoprisms and nanorods influenced by specific adsorption of halide ions. J. Phys. Chem. C 2007, 111, 1123–1130. [Google Scholar] [CrossRef]
- Chen, X.J.; Cheng, M.; Chen, D.; Wang, R.M. Shape-controlled synthesis of Co2P nanostructures and their application in supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 3892–3900. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.F.; Yuan, S.L.; Tian, Z.M.; Yin, S.Y.; He, J.H.; Liu, K.L.; Liu, L. One-pot synthesis of hollow nickel phosphide nanoparticles with tunable void sizes using triphenylphosphine. Mater. Lett. 2009, 63, 2283–2285. [Google Scholar] [CrossRef]
- Wu, D.; Dai, C.; Li, S.; Cheng, D. Shape-controlled synthesis of pdcu nanocrystals for formic acid oxidation. Chem. Lett. 2015, 44, 1101–1103. [Google Scholar] [CrossRef]
- Suo, Y.; Zhuang, L.; Lu, J. First-principles considerations in the design of Pd-alloy catalysts for oxygen reduction. Angew. Chem. Int. Ed. Engl. 2007, 46, 2862–2864. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Q.; Li, Y.; Han, M.; Gu, L.; Nan, C.; Bao, J.; Dai, Z. Five-fold twinned Pd2NiAg nanocrystals with increased surface Ni site availability to improve oxygen reduction activity. J. Am. Chem. Soc. 2015, 137, 2820–2823. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chong, H.; Li, P.; Xiang, J.; Fu, F.; Yang, S.; Yu, H.; Sheng, H.; Zhu, M. Pd-Ni alloy nanoparticles as effective catalysts for miyaura-heck coupling reactions. J. Phys. Chem. C 2015, 119, 11511–11515. [Google Scholar] [CrossRef]
- Han, S.-H.; Bai, J.; Liu, H.-M.; Zeng, J.-H.; Jiang, J.-X.; Chen, Y.; Lee, J.-M. One-pot fabrication of hollow and porous Pd-Cu alloy nanospheres and their remarkably improved catalytic performance for hexavalent chromium reduction. ACS Appl. Mater. Interfaces 2016, 8, 30948–30955. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.M.; Park, H.; Banu, M.; Kim, J.Y.; Youn, D.H.; Magesh, G.; Kim, W.Y.; Lee, J.S. Catalytic Co2 hydrogenation to formic acid over carbon nanotube-graphene supported PDNI alloy catalysts. RSC Adv. 2015, 5, 105560–105566. [Google Scholar] [CrossRef]
- Wang, Y.; Balbuena, P.B. Design of oxygen reduction bimetallic catalysts: Ab-initio-derived thermodynamic guidelines. J. Phys. Chem. B 2005, 109, 18902–18906. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Shi, X.; Zhang, Y.; Hong, C.; Wang, C.; Budzianowski, W.M.; Xue, D. Catalytic oxidation of hydroquinone in aqueous solution over bimetallic PdCo catalyst supported on carbon: Effect of interferents and electrochemical measurement. ACS Appl. Mater. Interfaces 2016, 8, 2994–3002. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Tomishige, K. Total hydrogenation of furan derivatives over silica-supported Ni-Pd alloy catalyst. Catal. Commun. 2010, 12, 154–156. [Google Scholar] [CrossRef]
- Massard, R.; Uzio, D.; Thomazeau, C.; Pichon, C.; Rousset, J.L.; Bertolini, J.C. Strained Pd overlayers on Ni nanoparticles supported on alumina and catalytic activity for buta-1,3-diene selective hydrogenation. J. Catal. 2007, 245, 133–143. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, D.; Zhao, P.; Niu, Z.; Peng, Q.; Li, Y. Monodispersed Pd-Ni nanoparticles: Composition control synthesis and catalytic properties in the Miyaura-Suzuki reaction. Inorg. Chem. 2011, 50, 2046–2048. [Google Scholar] [CrossRef] [PubMed]
- Son, S.U.; Jang, Y.; Park, J.; Na, H.B.; Park, H.M.; Yun, H.J.; Lee, J.; Hyeon, T. Designed synthesis of atom-economical Pd/Ni bimetallic nanoparticle-based catalysts for sonogashira coupling reactions. J. Am. Chem. Soc. 2004, 126, 5026–5027. [Google Scholar] [CrossRef] [PubMed]
- Demirci, U.B. Theoretical means for searching bimetallic alloys as anode electrocatalysts for direct liquid-feed fuel cells. J. Power Sources 2007, 173, 11–18. [Google Scholar] [CrossRef]
- Balanta, A.; Godard, C.; Claver, C. Pd nanoparticles for C-C coupling reactions. Chem. Soc. Rev. 2011, 40, 4973–4985. [Google Scholar] [CrossRef] [PubMed]
- Bumagin, N.A. High-turnover aminopyridine-based Pd-catalysts for Suzuki–Miyaura reaction in aqueous media. Catal. Commun. 2016, 79, 17–20. [Google Scholar] [CrossRef]
- Kim, M.; Kang, H.; Park, K.H. Pd nanoparticles supported on Fe3O4@amine-functionalized graphene composite and catalytic performance in sonogashira cross-coupling reactions. Catal. Commun. 2015, 72, 150–155. [Google Scholar] [CrossRef]
- Le, X.D.; Dong, Z.P.; Jin, Z.C.; Wang, Q.Q.; Ma, J.T. Suzuki–Miyaura cross-coupling reactions catalyzed by efficient and recyclable Fe3O4@SiO2@mSiO2-Pd(II) catalyst. Catal. Commun. 2014, 53, 47–52. [Google Scholar] [CrossRef]
- Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.M.; Polshettiwar, V. Nanocatalysts for Suzuki cross-coupling reactions. Chem. Soc. Rev. 2011, 40, 5181–5203. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.; Park, J.C.; Park, S.; Park, K.H. Rose-like Pd-Fe3O4 hybrid nanocomposite-supported Au nanocatalysts for tandem synthesis of 2-phenylindoles. Nanoscale 2015, 7, 8356–8360. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.; Kim, D.; Park, J.C.; Kim, J.W.; Park, S.; Lee, J.M.; Park, K.H. A new hybrid nanocatalyst based on Cu-doped Pd-Fe3O4 for tandem synthesis of 2-phenylbenzofurans. J. Mater. Chem. A 2015, 3, 20992–20998. [Google Scholar] [CrossRef]
- Lee, K.; Kang, S.W.; Lee, S.-U.; Park, K.-H.; Lee, Y.W.; Han, S.W. One-pot synthesis of monodisperse 5 nm Pd-Ni nanoalloys for electrocatalytic ethanol oxidation. ACS Appl. Mater. Interfaces 2012, 4, 4208–4214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jin, M.S.; Xiong, Y.J.; Lim, B.; Xia, Y.N. Shape-controlled synthesis of Pd nanocrystals and their catalytic applications. Acc. Chem. Res. 2013, 46, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, C.M.; Chen, H.; Su, D.S.; Zhang, B.S.; Cao, X.Z.; Wang, B.Y.; Wei, M.; Evans, D.G.; Duan, X. A surface defect-promoted Ni nanocatalyst with simultaneously enhanced activity and stability. Chem. Mater. 2013, 25, 1040–1046. [Google Scholar] [CrossRef]
- Xia, H.W.; Fu, Y.S.; He, G.Y.; Sun, X.Q.; Wang, X. Core-shell-like Ni-Pd nanoparticles supported on carbon black as a magnetically separable catalyst for green Suzuki–Miyaura coupling reactions. Appl. Catal. B Environ. 2017, 200, 39–46. [Google Scholar] [CrossRef]
- Rai, R.K.; Gupta, K.; Tyagi, D.; Mahata, A.; Behrens, S.; Yang, X.; Xu, Q.; Pathak, B.; Singh, S.K. Access to highly active Ni-Pd bimetallic nanoparticle catalysts for C-C coupling reactions. Catal. Sci. Technol. 2016, 6, 5567–5579. [Google Scholar] [CrossRef]






| Nanoparticles | Pd (wt %) | Fe (wt %) | Ni (wt %) |
|---|---|---|---|
| NPFNP-1 | 26.68 | 44.47 | 11.23 |
| NPFNP-2 | 27.88 | 42.65 | 12.36 |
| Pd-Fe3O4 | 28.5 | 51.11 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.; Kim, T.; Park, K.H. Fabrication of Crumpled Ball-Like Nickel Doped Palladium-Iron Oxide Hybrid Nanoparticles with Controlled Morphology as Effective Catalyst for Suzuki–Miyaura Coupling Reaction. Catalysts 2017, 7, 247. https://doi.org/10.3390/catal7090247
Jang S, Kim T, Park KH. Fabrication of Crumpled Ball-Like Nickel Doped Palladium-Iron Oxide Hybrid Nanoparticles with Controlled Morphology as Effective Catalyst for Suzuki–Miyaura Coupling Reaction. Catalysts. 2017; 7(9):247. https://doi.org/10.3390/catal7090247
Chicago/Turabian StyleJang, Seongwan, Taewoo Kim, and Kang Hyun Park. 2017. "Fabrication of Crumpled Ball-Like Nickel Doped Palladium-Iron Oxide Hybrid Nanoparticles with Controlled Morphology as Effective Catalyst for Suzuki–Miyaura Coupling Reaction" Catalysts 7, no. 9: 247. https://doi.org/10.3390/catal7090247
APA StyleJang, S., Kim, T., & Park, K. H. (2017). Fabrication of Crumpled Ball-Like Nickel Doped Palladium-Iron Oxide Hybrid Nanoparticles with Controlled Morphology as Effective Catalyst for Suzuki–Miyaura Coupling Reaction. Catalysts, 7(9), 247. https://doi.org/10.3390/catal7090247