Continuous-Flow Monolithic Silica Microreactors with Arenesulphonic Acid Groups: Structure–Catalytic Activity Relationships
Abstract
:1. Introduction
2. Results
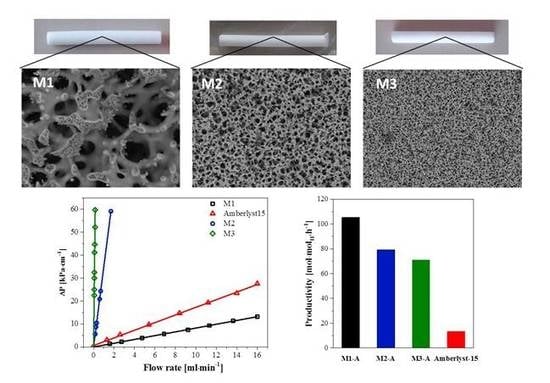
2.1. Characterization of the Silica Monoliths
2.2. Catalytic Performance
3. Materials and Methods
3.1. Monoliths Synthesis and Activation
3.2. Characterization of Materials
3.3. Catalytic Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Plumb, K. Continuous processing in the pharmaceutical industry—Changing the mind set. Chem. Eng. Res. Des. 2005, 83, 730–738. [Google Scholar] [CrossRef]
- Malet-Sanz, L.; Susanne, F. Continuous flow synthesis. A pharma perspective. J. Med. Chem. 2012, 55, 4062–4098. [Google Scholar] [CrossRef] [PubMed]
- Teoh, S.K.; Rathi, C.; Sharratt, P. Practical assessment methodology for converting fine chemicals processes from batch to continuous. Org. Process Res. Dev. 2016, 20, 414–431. [Google Scholar] [CrossRef]
- Stankiewicz, A. Process intensification in in-line monolithic reactor. Chem. Eng. Sci. 2001, 56, 359–364. [Google Scholar] [CrossRef]
- Ehrfeld, W.; Hessel, V.; Lowe, H. Microreactors: New Technology for Modern Chemistry; Willey-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Wirth, T. Microreactors in Organic Chemistry and Catalysis, 2nd ed.; Willey-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Peterson, D.S.; Rohr, T.; Svec, F.; Frechet, J.M.J. Enzymatic microreactor-on-a-chip: Protein mappingusing trypsin immobilized on porous polymer monoliths molded in channels of microfluidic devices. Anal. Chem. 2002, 74, 4081–4088. [Google Scholar] [CrossRef] [PubMed]
- Poupart, P.; Le Droumaguet, B.; Guerrouache, M.; Carbonnier, B. Copper nanoparticles supported on permeable monolith with carboxylic acid surface functinality: Stability and catalytic properties under reductive conditions. Mater. Chem. Phys. 2015, 163, 446–452. [Google Scholar] [CrossRef]
- Khalil, A.M.; Georgiadou, V.; Guerrouache, M.; Mahouche-Chergui, S.; Dendrinou-Samara, C.; Chehimi, M.M.; Carbonnier, B. Gold-decorated polymeric monoliths: In-situ vs. ex-situ immobilization strategies and flow through catalytic applications towards nitrophenol reduction. Polymer 2015, 77, 218–226. [Google Scholar] [CrossRef]
- El Kadib, A.; Chimenton, R.; Sachse, A.; Fajula, F.; Galarneau, A.; Coq, B. Functionalized inorganic monolithic microreactors for high productivity in fine chemicals catalytic synthesis. Angew. Chem. Int. Ed. 2009, 48, 4969–4972. [Google Scholar] [CrossRef] [PubMed]
- Sachse, A.; Galarneau, A.; Fajula, F.; Di Renzo, F.; Creux, P.; Coq, B. Functional silica monoliths with hierarchical uniform porosity as continuous flow catalytic reactors. Microporous Mesoporous Mater. 2011, 140, 58–68. [Google Scholar] [CrossRef]
- Siouffi, A.M. Silica gel-based monoliths prepared by the sol-gel method: Facts and figures. J. Chromatogr. A 2003, 1000, 801–818. [Google Scholar] [CrossRef]
- Nakanishi, K.; Minakuchi, H.; Soga, N.; Tanaka, N. Structure design of double-pore silica and its application to HPLC. J. Sol. Gel Sci. Technol. 1998, 13, 163–169. [Google Scholar] [CrossRef]
- Nakanishi, K.; Kobayashi, Y.; Amatani, T.; Hirao, K.; Kodaira, T. Spontaneous formation of hierarchical macro-mesoporous ethane-silica monolith. Chem. Mater. 2004, 16, 3652–3658. [Google Scholar] [CrossRef]
- Galarneau, A.; Iapichella, J.; Bonhomme, K.; Di Renzo, F.; Kooyman, P.; Terasaki, O.; Fajula, F. Controlling the morphology of mesostructured silicas by pseudomorphic transformation: A route towards applications. Adv. Funct. Mater. 2006, 16, 1657–1667. [Google Scholar] [CrossRef]
- Martin, T.; Galarneau, A.; Di Renzo, F.; Fajula, F.; Plee, D. Morphological control of MCM-41 by pseudomorphic synthesis. Angew. Chem. Int. Ed. 2002, 41, 2590–2592. [Google Scholar] [CrossRef]
- Na, K.; Jo, C.; Kim, J.; Cho, K.; Jung, J.; Seo, Y.; Messinger, R.J.; Chmelka, B.F.; Ryoo, R. Directing zeolite structures into hierarchically nanoporous architectures. Science 2011, 333, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Sun, M.H.; Rooke, J.C.; Chen, L.H.; Su, B.L. Synthesis and applications of hierarchically porous catalysts. Chin. J. Catal. 2013, 34, 22–47. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Froba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef] [PubMed]
- Koreniuk, A.; Maresz, K.; Odrozek, K.; Jarzębski, A.B.; Mrowiec-Białoń, J. Highly effective continuous-flow monolithic silica microreactors for acid catalyzed processes. Appl. Catal. A 2015, 489, 203–208. [Google Scholar] [CrossRef]
- Szymańska, K.; Pudło, W.; Mrowiec-Białoń, J.; Czardybon, A.; Kocurek, J.; Jarzębski, A.B. Immobilization of invertase on silica monoliths with hierarchical pore structure to obtain continuous flow enzymatic microreactors of high performance. Microporous Mesoporous Mater. 2013, 170, 75–82. [Google Scholar] [CrossRef]
- Kawakami, K.; Sera, Y.; Sakai, S.; Ono, T.; Ijima, H. Development and characterization of a silica monolith immobilized enzyme micro-bioreactor. Ind. Eng. Chem. Res. 2005, 44, 236–240. [Google Scholar] [CrossRef]
- Koreniuk, A.; Maresz, K.; Mrowiec-Białoń, J. Supported zirconium-based continuous-flow microreactor for effective Meerwein-Ponndorf-Verley reduction of cyclohexanone. Catal. Commun. 2015, 64, 48–51. [Google Scholar] [CrossRef]
- Cheung, H.; Tanke, R.S.; Torrence, G.P. Ullman's Encyclopedia of Industrial Chemistry; Willey-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Szymańska, K.; Odrozek, K.; Zniszczoł, A.; Pudło, W.; Jarzębski, A.B. A novel hierarchically structured siliceous packing to boost the performance of rotating bed enzymatic reactors. Chem. Eng. J. 2017, 315, 18–24. [Google Scholar] [CrossRef]
- Smått, J.H.; Schunk, S.; Linden, M. Versatile double-templating synthesis route to silica monoliths exhibiting a multimodal hierarchical porosity. Chem. Mater. 2003, 15, 2354–2361. [Google Scholar] [CrossRef]
- Pudło, W.; Gawlik, W.; Mrowiec-Białoń, J.; Buczek, T.; Malinowski, J.J.; Jarzębski, A.B. Materials with multimodal hierarchical porosity. Inzynieria Chemiczna i Procesowa 2006, 27, 177–185. [Google Scholar]
- Szymańska, K.; Pietrowska, M.; Kocurek, J.; Maresz, K.; Koreniuk, A.; Mrowiec-Białoń, J.; Widłak, P.; Magner, E.; Jarzębski, A. Low back-pressure hierarchically structured multichannel microfluidic bioreactors for rapid protein digestion—Proof of concept. Chem. Eng. J. 2016, 287, 148–154. [Google Scholar] [CrossRef]
- Mrowiec-Białoń, J. Determination of hydroxyls density in the silica-mesostructured cellular foams by thermogravimetry. Thermochim. Acta 2006, 443, 49–52. [Google Scholar] [CrossRef]
- Stawicka, K.; Diaz-Alvarez, A.E.; Calvino-Casilda, V.; Trejda, M.; Banares, M.A.; Ziolek, M. The role of Brönsted and Lewis acid sites in acetalization of glycerol over modified mesoporous cellular foams. J. Phys. Chem. C 2016, 120, 16699–16711. [Google Scholar] [CrossRef]
- Datka, J.; Turek, A.M.; Jehng, J.M.; Wachs, I.E. Acidic properties of supported niobium oxide catalysts—An infrared-spectroscopy investigation. J. Catal. 1992, 135, 186–199. [Google Scholar] [CrossRef]
- Sachse, A.; Hulea, V.; Finiels, A.; Coq, B.; Fajula, F.; Galarneau, A. Alumina-grafted macro-/mesoporous silica monoliths as continuous flow microreactors for the Diels-Alder reaction. J. Catal. 2012, 287, 62–67. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Zeyer, K.P.; Jacobs, T.; Kienle, A. Miniaturized systems for homogeneously and heterogeneously catalyzed liquid-phase esterification reaction. Ind. Eng. Chem. Res. 2007, 46, 5271–5277. [Google Scholar] [CrossRef]
- Jermy, B.R.; Pandurangan, A. A highly efficient catalyst for the esterification of acetic acid using n-butyl alcohol. J. Mol. Catal. A Chem. 2005, 237, 146–154. [Google Scholar] [CrossRef]
- Peters, N.E.; Benes, N.E.; Holmen, A.; Keurentjes, J.T.F. Comparison of commercial solid acid catalysts for the esterification of acetic acid with butanol. Appl. Catal. A 2006, 297, 182–188. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.; Joyner, L.; Halenda, P. The determination of pore volume and area distributions in porous substances. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]






| Sample | SBET (m2·g−1) | Vpmes (cm3·g−1) | VT (cm3·g−1) | Dmac (μm) | Dmes 1 (nm) | H+ (mmol·g−1) | A200/A150 4 |
|---|---|---|---|---|---|---|---|
| M1 | 328 | 1.15 | 4 | 30–50 | 2.5/20 | 0.65 | 0.9 |
| (245) 2 | (0.91) | (3.72) | (30–50) | (20) | |||
| M2 | 413 | 1.12 | 3.27 | 4–6 | 15 | 0.85 | 0.9 |
| (316) | (0.91) | 3.01 | 4–6 | (14.9) | |||
| M3 | 575 | 1.04 | 2.98 | 1.3 | 8.7 | 0.97 | 0.8 |
| (427) | (0.74) | 2.65 | 1.3 | (7.9) | |||
| Amberlyst | 40 | 0.33 | - | - | 31 | 4.7 3 | n/a |
| Sample | Permeability K·1012 (m2) |
|---|---|
| M1 | 11.3 |
| M2 | 0.27 |
| M3 | 0.025 |
| Amberlyst 15 | 5.4 |
| Sample | Conversion (%) | τ (min) | Productivity (mol·kgcat−1·h−1) | Productivity (mol·molH+−1·h−1) |
|---|---|---|---|---|
| M1-A | 42 | 10 | 68.4 | 105.2 |
| M2-A | 41 | 8 | 67.2 | 79.2 |
| M3-A | 42 | 7.5 | 68.4 | 70.8 |
| Amberlyst 15 | 38 | 4.2 | 61.8 | 13.2 |
| Entry | Catalyst | Conversion (%) | Productivity (mol·kgcat−1·h−1) | Temp. (°C) | Time (min) | Ref. |
|---|---|---|---|---|---|---|
| 1 | M1-A | 42 | 68.4 | 75 | 10 2 | This work |
| 2 | Amberlyst 15 1 | 70 | 30 | 80 | 10 2 | [33] |
| 3 | Al-MCM-41 | 50 | 29.4 | 150 | 360 | [34] |
| 4 | H-USY-20 | 35 | 13.8 | 75 | 360 | [35] |
| 5 | Smopex-101 | 65 | 37.8 | 75 | 360 | [35] |
| Sample | TEOS (cm3) | PEG (g) | H2O (cm3) | HNO3 (cm3) | CTAB (g) | Aging (Days) | Ammonia Treatment | ||
|---|---|---|---|---|---|---|---|---|---|
| Conc. (M) | Time (h) | Temp. (°C) | |||||||
| M1 | 26.1 | 2.73 | 30.10 | 2.18 | 1.2 | 7 | 1 | 8 | 90 |
| M2 | 26.1 | 3.31 | 31.44 | 2.04 | 0 | 3 | 1 | 24 | 80 |
| M3 | 26.1 | 3.32 | 32.05 | 2.39 | 0 | 3 | 0.1 | 20 | 40 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciemięga, A.; Maresz, K.; Malinowski, J.J.; Mrowiec-Białoń, J. Continuous-Flow Monolithic Silica Microreactors with Arenesulphonic Acid Groups: Structure–Catalytic Activity Relationships. Catalysts 2017, 7, 255. https://doi.org/10.3390/catal7090255
Ciemięga A, Maresz K, Malinowski JJ, Mrowiec-Białoń J. Continuous-Flow Monolithic Silica Microreactors with Arenesulphonic Acid Groups: Structure–Catalytic Activity Relationships. Catalysts. 2017; 7(9):255. https://doi.org/10.3390/catal7090255
Chicago/Turabian StyleCiemięga, Agnieszka, Katarzyna Maresz, Janusz J. Malinowski, and Julita Mrowiec-Białoń. 2017. "Continuous-Flow Monolithic Silica Microreactors with Arenesulphonic Acid Groups: Structure–Catalytic Activity Relationships" Catalysts 7, no. 9: 255. https://doi.org/10.3390/catal7090255
APA StyleCiemięga, A., Maresz, K., Malinowski, J. J., & Mrowiec-Białoń, J. (2017). Continuous-Flow Monolithic Silica Microreactors with Arenesulphonic Acid Groups: Structure–Catalytic Activity Relationships. Catalysts, 7(9), 255. https://doi.org/10.3390/catal7090255