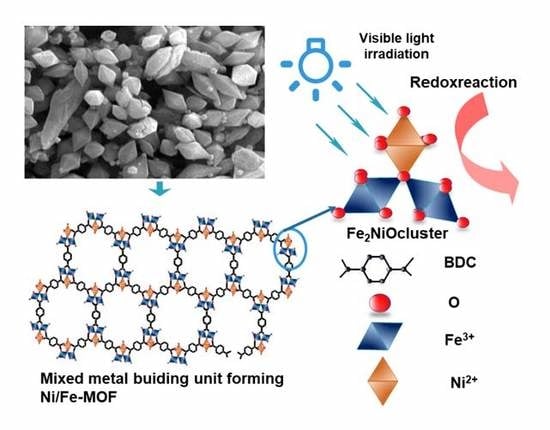
Effective Photocatalytic Activity of Mixed Ni/Fe-Base Metal-Organic Framework under a Compact Fluorescent Daylight Lamp
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Properties of MIL-53(Fe) and Ni-Doped MIL-53(Fe)
2.1.1. XRD Analysis
2.1.2. FT-IR Spectra
2.1.3. Raman Spectra
2.1.4. FE-SEM/EDS Analysis
2.1.5. XPS Spectra
2.1.6. N2 Adsorption/Desorption
2.1.7. UV-Vis Spectra
2.1.8. PL Spectroscopy
2.2. Photocatalytic Activities
2.2.1. RhB Removal by MIL-53(Fe) and Ni-MIL-53(Fe)
2.2.2. Effect of Initial Dye Concentration, Initial Solution pH, and the Molar Ratio of Ni2+/Fe3+ on the Degradation of RhB
3. Experimental
3.1. Materials
3.2. Preparation of Catalysts
3.3. Catalyst Characterization
3.4. Photocatalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tranchemontagne, D.J.; Mendoza-Cortés, J.L.; O’Keeffe, M.; Yaghi, O.M. Secondary building units, nets and bonding in the chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef] [PubMed]
- Hamon, L.; Serre, C.; Devic, T.; Loiseau, T.; Millange, F.; Férey, G.; De Weireld, G. Comparative study of hydrogen sulfide adsorption in the MIL-53(Al, Cr, Fe), MIL-47(V), MIL-100(Cr), and MIL-101(Cr) metal-organic frameworks at room temperature. J. Am. Chem. Soc. 2009, 131, 8775–8777. [Google Scholar] [CrossRef] [PubMed]
- Devic, T.; Salles, F.; Bourrelly, S.; Moulin, B.; Maurin, G.; Horcajada, P.; Serre, C.; Vimont, A.; Lavalley, J.C.; Leclerc, H.; et al. Effect of the organic functionalization of flexible MOFs on the adsorption of CO2. J. Mater. Chem. 2012, 22, 10266–10273. [Google Scholar] [CrossRef]
- Vu, T.A.; Le, G.H.; Dao, C.D.; Dang, L.Q.; Nguyen, K.T.; Nguyen, Q.K.; Dang, P.T.; Tran, H.T.K.; Duong, Q.T.; Nguyen, T.V.; et al. Arsenic removal from aqueous solutions by adsorption using novel MIL-53(Fe) as a highly efficient adsorbent. RSC Adv. 2015, 5, 5261–5268. [Google Scholar] [CrossRef]
- Jia, J.; Xu, F.; Long, Z.; Hou, X.; Sepaniak, M.J. Metal–organic framework MIL-53(Fe) for highly selective and ultrasensitive direct sensing of MeHg+. Chem. Commun. 2013, 49, 4670. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhai, M.; Guan, W.; Liu, J.; Liu, Z.; Damirin, A. Controllable synthesis of a smart multifunctional nanoscale metal-organic framework for magnetic resonance/optical imaging and targeted drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Bach, L.G.; Van Tran, T.; Nguyen, T.D.; Van Pham, T.; Do, S.T. Enhanced adsorption of methylene blue onto graphene oxide-doped XFe2O4(X = Co, Mn, Ni) nanocomposites: Kinetic, isothermal, thermodynamic and recyclability studies. Res. Chem. Intermed. 2018, 44, 1661–1687. [Google Scholar] [CrossRef]
- Nhung, N.T.H.; Quynh, B.T.P.; Thao, P.T.T.; Bich, H.N.; Giang, B.L. Pretreated Fruit Peels as Adsorbents for Removal of Dyes from Water. IOP Conf. Ser. Earth Environ. Sci. 2018, 159, 012015. [Google Scholar] [CrossRef]
- Kim, D.W.; Bach, L.G.; Hong, S.S.; Park, C.; Lim, K.T. A Facile Route towards the Synthesis of Fe3O4/Graphene Oxide Nanocomposites for Environmental Applications. Mol. Cryst. Liq. Cryst. 2014. [Google Scholar] [CrossRef]
- Van Thuan, T.; Ho, V.T.T.; Trinh, N.D.; Thuong, N.T.; Quynh, B.T.P.; Bach, L.G. Facile one-spot synthesis of highly porous KOH-activated carbon from rice husk: Response surface methodology approach. Carbon Sci. Technol. 2016, 8, 63–69. [Google Scholar]
- Trinh, N.D.; Hong, S.-S. Photocatalytic Decomposition of Methylene Blue Over MIL-53 (Fe) Prepared Using Microwave-Assisted Process Under Visible Light Irradiation. J. Nanosci. Nanotechnol. 2015, 15, 5450–5454. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Im, J.H.; Kim, T.; Lee, K.; Park, C.R. MOF-derived ZnO and ZnO@C composites with high photocatalytic activity and adsorption capacity. J. Hazard. Mater. 2011, 186, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Du, J.J.; Yuan, Y.P.; Sun, J.X.; Peng, F.M.; Jiang, X.; Qiu, L.G.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. New photocatalysts based on MIL-53 metal-organic frameworks for the decolorization of methylene blue dye. J. Hazard. Mater. 2011, 190, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ai, L.; Jiang, J. Solvothermal synthesis of MIL-53(Fe) hybrid magnetic composites for photoelectrochemical water oxidation and organic pollutant photodegradation under visible light. J. Mater. Chem. A 2015, 3, 3074–3081. [Google Scholar] [CrossRef]
- Alhamami, M.; Doan, H.; Cheng, C.H. A review on breathing behaviors of metal-organic-frameworks (MOFs) for gas adsorption. Materials 2014, 7, 3198–3250. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Commercial metal-organic frameworks as heterogeneous catalysts. Chem. Commun. 2012, 48, 11275–11288. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Zhang, C.; Li, L.; Jiang, J. Iron terephthalate metal-organic framework: Revealing the effective activation of hydrogen peroxide for the degradation of organic dye under visible light irradiation. Appl. Catal. B Environ. 2014, 148–149, 191–200. [Google Scholar] [CrossRef]
- Lionet, Z.; Kamata, Y.; Nishijima, S.; Toyao, T.; Kim, T.-H.; Horiuchi, Y.; Lee, S.W.; Matsuoka, M. Water oxidation reaction promoted by MIL-101(Fe) photoanode under visible light irradiation. Res. Chem. Intermed. 2018, 44, 4755–4764. [Google Scholar] [CrossRef]
- Qu, L.-L.; Wang, J.; Xu, T.-Y.; Chen, Q.-Y.; Chen, J.-H.; Shi, C.-J. Iron(iii)-based metal–organic frameworks as oxygen-evolving photocatalysts for water oxidation. Sustain. Energy Fuels 2018, 2, 2109–2114. [Google Scholar] [CrossRef]
- Wang, D.; Li, Z. Iron-based metal–organic frameworks (MOFs) for visible-light-induced photocatalysis. Res. Chem. Intermed. 2017, 43, 5169–5186. [Google Scholar] [CrossRef]
- Wang, D.; Albero, J.; García, H.; Li, Z. Visible-light-induced tandem reaction of o-aminothiophenols and alcohols to benzothiazoles over Fe-based MOFs: Influence of the structure elucidated by transient absorption spectroscopy. J. Catal. 2017, 349, 156–162. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Toyao, T.; Miyahara, K.; Zakary, L.; Do Van, D.; Kamata, Y.; Kim, T.-H.; Lee, S.W.; Matsuoka, M. Visible-light-driven photocatalytic water oxidation catalysed by iron-based metal–organic frameworks. Chem. Commun. 2016, 52, 5190–5193. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Deng, G.; Lu, W.; Pang, S.; Hu, X. Deposition of CdS nanoparticles on MIL-53(Fe) metal-organic framework with enhanced photocatalytic degradation of RhB under visible light irradiation. Appl. Surf. Sci. 2017, 410, 401–413. [Google Scholar] [CrossRef]
- Zhou, Y.; Mao, Z.; Wang, W.; Yang, Z.; Liu, X. In-Situ Fabrication of Graphene Oxide Hybrid Ni-Based Metal-Organic Framework (Ni-MOFs@GO) with Ultrahigh Capacitance as Electrochemical Pseudocapacitor Materials. ACS Appl. Mater. Interfaces 2016, 8, 28904–28916. [Google Scholar] [CrossRef] [PubMed]
- Panda, R.; Rahut, S.; Basu, J.K. Preparation of a Fe2O3/MIL-53(Fe) composite by partial thermal decomposition of MIL-53(Fe) nanorods and their photocatalytic activity. RSC Adv. 2016, 6, 80981–80985. [Google Scholar] [CrossRef]
- Lou, X.; Hu, H.; Li, C.; Hu, X.; Li, T.; Shen, M.; Chen, Q.; Hu, B. Capacity control of ferric coordination polymers by zinc nitrate for lithium-ion batteries. RSC Adv. 2016, 6, 86126–86130. [Google Scholar] [CrossRef]
- Vuong, G.T.; Pham, M.H.; Do, T.O. Synthesis and engineering porosity of a mixed metal Fe2Ni MIL-88B metal-organic framework. Dalt. Trans. 2013, 42, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Vuong, G.-T.; Pham, M.-H.; Do, T.-O. Direct synthesis and mechanism of the formation of mixed metal Fe2Ni-MIL-88B. CrystEngComm 2013, 15, 9694. [Google Scholar] [CrossRef]
- Pham, M.-H.; Dinh, C.-T.; Vuong, G.-T.; Ta, N.-D.; Do, T.-O. Visible light induced hydrogen generation using a hollow photocatalyst with two cocatalysts separated on two surface sides. Phys. Chem. Chem. Phys. 2014, 16, 5937. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, M.; Li, K.; Han, Y.; Zuo, Y.; Chai, F.; Song, C.; Zhang, G.; Guo, X. Synthesis of Fe/M (M = Mn, Co, Ni) bimetallic metal organic frameworks and their catalytic activity for phenol degradation under mild conditions. Inorg. Chem. Front. 2017, 4, 144–153. [Google Scholar] [CrossRef]
- Díaz-Gallifa, P.; Fabelo, O.; Pasán, J.; Cañadillas-Delgado, L.; Lloret, F.; Julve, M.; Ruiz-Pérez, C. Two-dimensional 3d-4f heterometallic coordination polymers: Syntheses, crystal structures, and magnetic properties of six new Co(II)-Ln(III) compounds. Inorg. Chem. 2014, 53, 6299–6308. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.A.; Le, G.H.; Dao, C.D.; Dang, L.Q.; Nguyen, K.T.; Dang, P.T.; Tran, H.T.K.; Duong, Q.T.; Nguyen, T.V.; Lee, G.D. Isomorphous substitution of Cr by Fe in MIL-101 framework and its application as a novel heterogeneous photo-Fenton catalyst for reactive dye degradation. RSC Adv. 2014, 40, 41185–41194. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, P.; Xiao, S.; Wang, W.; Zhang, D.; Li, H. Microwave-assisted synthesis of Ag-doped MOFs-like organotitanium polymer with high activity in visible-light driven photocatalytic NO oxidization. Appl. Catal. B Environ. 2015, 172–173, 46–51. [Google Scholar] [CrossRef]
- Han, Y.; Liu, M.; Li, K.; Sun, Q.; Zhang, W.; Song, C.; Zhang, G.; Conrad Zhang, Z.; Guo, X. In situ synthesis of titanium doped hybrid metal-organic framework UiO-66 with enhanced adsorption capacity for organic dyes. Inorg. Chem. Front. 2017, 4, 1870–1880. [Google Scholar] [CrossRef]
- Vinh, N.H.; Long Giang, B.; Sy Trung, D.; Thuong, N.T.; Trinh, N.D. Photoluminescence Properties of Eu-Doped MIL-53(Fe) Obtained by Solvothermal Synthesis. J. Nanosci. Nanotechnol. 2019, 19, 1148–1150. [Google Scholar]
- Chi, Y.X.; Niu, S.Y.; Jin, J. Syntheses, structures and photophysical properties of a series of Zn-Ln coordination polymers (Ln = Nd, Pr, Sm, Eu, Tb, Dy). Inorg. Chim. Acta 2009, 362, 3821–3828. [Google Scholar] [CrossRef]
- Tahir, M. Synergistic effect in MMT-dispersed Au/TiO2 monolithic nanocatalyst for plasmon-absorption and metallic interband transitions dynamic CO2 photo-reduction to CO. Appl. Catal. B Environ. 2017, 219, 329–343. [Google Scholar] [CrossRef]
- Tahir, M. Ni/MMT-promoted TiO2 nanocatalyst for dynamic photocatalytic H2 and hydrocarbons production from ethanol-water mixture under UV-light. Int. J. Hydrogen Energy 2017, 42, 28309–28326. [Google Scholar] [CrossRef]
- Haque, E.; Khan, N.; Park, H.J.; Jhung, S.H. Synthesis of a metal-organic framework material, iron terephthalate, by ultrasound, microwave, and conventional electric heating: A kinetic study. Chem. A Eur. J. 2010, 16, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Pu, M.; Guan, Z.; Ma, Y.; Wan, J.; Wang, Y.; Brusseau, M.L.; Chi, H. Synthesis of iron-based metal-organic framework MIL-53 as an efficient catalyst to activate persulfate for the degradation of Orange G in aqueous solution. Appl. Catal. A Gen. 2018, 549, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, H.; Jiang, F. In-situ ethylenediamine-assisted synthesis of a magnetic iron-based metal-organic framework MIL-53(Fe) for visible light photocatalysis. J. Colloid Interface Sci. 2017, 494, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef] [Green Version]
- Nakamoto, K. Applications in Organometallic Chemistry. In Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley & Sons, Inc.: Hoboken, NJ, USA; pp. 275–331. ISBN 9780470405888.
- Gao, Y.; Li, S.; Li, Y.; Yao, L.; Zhang, H. Accelerated photocatalytic degradation of organic pollutant over metal-organic framework MIL-53(Fe) under visible LED light mediated by persulfate. Appl. Catal. B Environ. 2017, 202, 165–174. [Google Scholar] [CrossRef]
- Vu, T.A.; Le, G.H.; Vu, H.T.; Nguyen, K.T.; Quan, T.T.T.; Nguyen, Q.K.; Tran, H.T.K.; Dang, P.T.; Vu, L.D.; Lee, G.D. Highly photocatalytic activity of novel Fe-MIL-88B/GO nanocomposite in the degradation of reactive dye from aqueous solution. Mater. Res. Express 2017, 4, 035038. [Google Scholar] [CrossRef]
- Islam, M.R.; Bach, L.G.; Vo, T.S.; Tran, T.N.; Lim, K.T. Nondestructive chemical functionalization of MWNTs by poly(2-dimethylaminoethyl methacrylate) and their conjugation with CdSe quantum dots: Synthesis, properties, and cytotoxicity studies. Appl. Surf. Sci. 2013. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Molchan, I.S.; Thompson, G.E.; Skeldon, P.; Lindsay, R.; Walton, J.; Kouvelos, E.; Romanos, G.E.; Falaras, P.; Kontos, A.G.; Arfanis, M.; et al. Microscopic study of the corrosion behaviour of mild steel in ionic liquids for CO2 capture applications. RSC Adv. 2015, 5, 35181–35194. [Google Scholar] [CrossRef]
- Lian, K.K.; Kirk, D.W.; Thorpe, S.J. Investigation of a “Two-State” Tafel Phenomenon for the Oxygen Evolution Reaction on an Amorphous Ni-Co Alloy. J. Electrochem. Soc. 1995, 142, 3704–3712. [Google Scholar] [CrossRef]
- Siang, T.J.; Bach, L.G.; Singh, S.; Truong, Q.D.; Ho, V.T.T.; Huy Phuc, N.H.; Alenazey, F.; Vo, D.V.N. Methane bi-reforming over boron-doped Ni/SBA-15 catalyst: Longevity evaluation. Int. J. Hydrogen Energy 2018. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef] [Green Version]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Flexible porous metal-organic frameworks for a controlled drug delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.B.; Yavari, A.; Hatfield, W.E.; Sethulekshmi, C.N. Magnetic and spectroscopic properties of some heterotrinuclear basic acetates of chromium(III), iron(III), and divalent metal ions. J. Chem. Soc. Dalt. Trans. 1985, 2509–2520. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Liu, B.; Zhao, Q.; Chen, G. Hexagonal microspindle of NH2-MIL-101(Fe) metal-organic frameworks with visible-light-induced photocatalytic activity for the degradation of toluene. RSC Adv. 2016, 6, 4289–4295. [Google Scholar] [CrossRef]
- Nguyen, M.T.H.; Nguyen, Q.T. Efficient refinement of a metal-organic framework MIL-53(Fe) by UV-vis irradiation in aqueous hydrogen peroxide solution. J. Photochem. Photobiol. A Chem. 2014, 288, 55–59. [Google Scholar] [CrossRef]
- Ling, S.; Slater, B. Unusually Large Band Gap Changes in Breathing Metal-Organic Framework Materials. J. Phys. Chem. C 2015, 119, 16667–16677. [Google Scholar] [CrossRef]
- Hou, Y.; Yuan, H.; Chen, H.; Feng, J.; Ding, Y.; Li, L. Preparation of La3+/Zn2+-doped BiVO4 nanoparticles and its enhanced visible photocatalytic activity. Appl. Phys. A 2017, 123, 611. [Google Scholar] [CrossRef]
- Dutta, D.P.; Ballal, A.; Chopade, S.; Kumar, A. A study on the effect of transition metal (Ti4+, Mn2+, Cu2+ and Zn2+)-doping on visible light photocatalytic activity of Bi2MoO6 nanorods. J. Photochem. Photobiol. A Chem. 2017, 346, 105–112. [Google Scholar] [CrossRef]
- Jin, A.Z.; Dong, W.; Yang, M.; Wang, J.; Wang, G.; Jin, Z.; Dong, W.; Yang, M.; Wang, J.; Wang, G. One-pot Preparation of Hierarchical Nanosheet-Constructed Fe3O4/MIL-88B (Fe) Magnetic Microspheres with High Efficiency Photocatalytic Degradation of Dye. ChemCatChem 2016, 22, 3510–3517. [Google Scholar] [CrossRef]
- Liang, R.; Luo, S.; Jing, F.; Shen, L.; Qin, N.; Wu, L. A simple strategy for fabrication of Pd@MIL-100(Fe) nanocomposite as a visible-light-driven photocatalyst for the treatment of pharmaceuticals and personal care products (PPCPs). Appl. Catal. B Environ. 2015, 176–177, 240–248. [Google Scholar] [CrossRef]
- Laurier, K.G.M.; Vermoortele, F.; Ameloot, R.; De Vos, D.E.; Hofkens, J.; Roeffaers, M.B.J. Iron(III)-based metal-organic frameworks as visible light photocatalysts. J. Am. Chem. Soc. 2013, 135, 14488–14491. [Google Scholar] [CrossRef] [PubMed]
- Martínez, F.; Leo, P.; Orcajo, G.; Díaz-García, M.; Sanchez-Sanchez, M.; Calleja, G. Sustainable Fe-BTC catalyst for efficient removal of mehylene blue by advanced fenton oxidation. Catal. Today 2018, 313, 6–11. [Google Scholar] [CrossRef]
- Gao, C.; Chen, S.; Quan, X.; Yu, H.; Zhang, Y. Enhanced Fenton-like catalysis by iron-based metal organic frameworks for degradation of organic pollutants. J. Catal. 2017, 356, 125–132. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Gao, B.; Chen, Y.; Lin, B. Hydrothermal synthesis of In2O3-loaded BiVO4 with exposed {010}{110} facets for enhanced visible-light photocatalytic activity. Mater. Res. Bull. 2017, 87, 114–118. [Google Scholar] [CrossRef]
- Sotnik, S.A.; Polunin, R.A.; Kiskin, M.A.; Kirillov, A.M.; Dorofeeva, V.N.; Gavrilenko, K.S.; Eremenko, I.L.; Novotortsev, V.M.; Kolotilov, S.V. Heterometallic coordination polymers assembled from trigonal trinuclear Fe2Ni-pivalate blocks and polypyridine spacers: Topological diversity, sorption, and catalytic properties. Inorg. Chem. 2015, 54, 5169–5181. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Håkansson, M.; Suomi, J.; Ala-Kleme, T.; Kulmala, S. Cathodic electrochemiluminescence of lucigenin at disposable oxide-coated aluminum electrodes. J. Electroanal. Chem. 2006, 591, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Van Thuan, T.; Quynh, B.T.P.; Nguyen, T.D.; Ho, V.T.T.; Bach, L.G. Response surface methodology approach for optimization of Cu2+, Ni2+ and Pb2+ adsorption using KOH-activated carbon from banana peel. Surf. Interfaces 2017, 6, 209–217. [Google Scholar] [CrossRef]
- Van Tran, T.; Bui, Q.T.P.; Nguyen, T.D.; Le, N.T.H.; Bach, L.G. A comparative study on the removal efficiency of metal ions (Cu2+, Ni2+, and Pb2+) using sugarcane bagasse-derived ZnCl2-activated carbon by the response surface methodology. Adsorpt. Sci. Technol. 2017, 35, 72–85. [Google Scholar] [CrossRef]
- Van Tran, T.; Bui, Q.T.P.; Nguyen, T.D.; Thanh Ho, V.T.; Bach, L.G. Application of response surface methodology to optimize the fabrication of ZnCl2-activated carbon from sugarcane bagasse for the removal of Cu2+. Water Sci. Technol. 2017, 75, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Dinh, C.-T.; Nguyen, T.-D.; Kleitz, F.; Do, T.-O. Large-scale synthesis of uniform silver orthophosphate colloidal nanocrystals exhibiting high visible light photocatalytic activity. Chem. Commun. 2011, 47, 7797–7799. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cohen, S.M. Photocatalytic metal-organic frameworks for the aerobic oxidation of arylboronic acids. Chem. Commun. 2015, 51, 9880–9883. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Sin, J.C.; Abdullah, A.Z.; Mohamed, A.R. Efficient photodegradation of resorcinol with Ag2O/ZnO nanorods heterostructure under a compact fluorescent lamp irradiation. Chem. Pap. 2013, 67, 1277–1284. [Google Scholar] [CrossRef]











| Samples | Specific Surface Area (m2/g) | Micropore Volume (×10−3 cm3/g) | Mesopore Volume (×10−3 cm3/g) | Average Pore Width (nm) |
|---|---|---|---|---|
| MIL-53(Fe)·DMF | 300 | 128 | 97 | 13 |
| MIL-53(Fe)·H2O | 158 | 65 | 59 | 11 |
| Ni/Fe-MOF-0.3·DMF | 480 | 212 | 128 | 8 |
| Ni/Fe-MOF-0.3·H2O | 247 | 94 | 271 | 13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.H.; Nguyen, T.D.; Bach, L.G.; Hoang, T.; Bui, Q.T.P.; Tran, L.D.; Nguyen, C.V.; Vo, D.-V.N.; Do, S.T. Effective Photocatalytic Activity of Mixed Ni/Fe-Base Metal-Organic Framework under a Compact Fluorescent Daylight Lamp. Catalysts 2018, 8, 487. https://doi.org/10.3390/catal8110487
Nguyen VH, Nguyen TD, Bach LG, Hoang T, Bui QTP, Tran LD, Nguyen CV, Vo D-VN, Do ST. Effective Photocatalytic Activity of Mixed Ni/Fe-Base Metal-Organic Framework under a Compact Fluorescent Daylight Lamp. Catalysts. 2018; 8(11):487. https://doi.org/10.3390/catal8110487
Chicago/Turabian StyleNguyen, Vinh Huu, Trinh Duy Nguyen, Long Giang Bach, Thai Hoang, Quynh Thi Phuong Bui, Lam Dai Tran, Chuong V. Nguyen, Dai-Viet N. Vo, and Sy Trung Do. 2018. "Effective Photocatalytic Activity of Mixed Ni/Fe-Base Metal-Organic Framework under a Compact Fluorescent Daylight Lamp" Catalysts 8, no. 11: 487. https://doi.org/10.3390/catal8110487
APA StyleNguyen, V. H., Nguyen, T. D., Bach, L. G., Hoang, T., Bui, Q. T. P., Tran, L. D., Nguyen, C. V., Vo, D. -V. N., & Do, S. T. (2018). Effective Photocatalytic Activity of Mixed Ni/Fe-Base Metal-Organic Framework under a Compact Fluorescent Daylight Lamp. Catalysts, 8(11), 487. https://doi.org/10.3390/catal8110487