The Influence of O/S Exchange on the Biocatalytical Activity of Benzisoselenazol-3(2H)-ones
Abstract
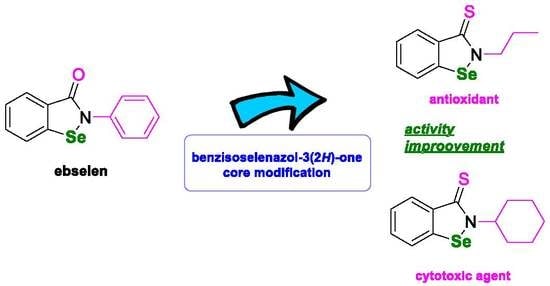
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Catalytical and Biological Activity Evaluation
3. Materials and Methods
3.1. Chemistry
3.1.1. General
3.1.2. Synthesis of o-iodobenzamides 4a, 4b and 4d
N-ethyl-o-iodobenzamide 4a
N-propyl-o-iodobenzamide 4b
N-hexyl-o-iodobenzamide 4d
3.1.3. Synthesis of o-iodobenzthioamides 5a–5f
N-ethyl-o-iodobenzthioamide 5a
N-propyl-o-iodobenzthioamide 5b
N-butyl-o-iodobenzthioamide 5c
N-hexyl-o-iodobenzthioamide 5d
N-(3-methylbutyl)-o-iodobenzthioamide 5e
N-cyclohexyl-o-iodobenzthioamide 5f
3.1.4. Synthesis of Benzisoselenazol-3(2H)-ones 6a, 6b and 6d
N-ethyl-1,2-benzisoselenazol-3(2H)-one 6a
N-propyl-1,2-benzisoselenazol-3(2H)-one 6b
N-hexyl-1,2-benzisoselenazol-3(2H)-one 6d
3.1.5. Synthesis of Benzisoselenazol-3(2H)-thiones 7a–7f
N-ethyl-1,2-benzisoselenazol-3(2H)-thione 7a
N-propyl-1,2-benzisoselenazol-3(2H)-thione 7b
N-butyl-1,2-benzisoselenazol-3(2H)-thione 7c
N-hexyl-1,2-benzisoselenazol-3(2H)-thione 7d
N-(3-methylbutyl)-1,2-benzisoselenazol-3(2H)-thione 7e
N-cyclohexyl-1,2-benzisoselenazol-3(2H)-thione 7f
3.2. Biological Activity Evaluation
3.2.1. Antioxidant Activity Assay
3.2.2. SRB viability Assay
Cell Culture
SRB Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Weekley, C.M.; Harris, H.H. Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 2013, 42, 8870–8894. [Google Scholar] [CrossRef] [PubMed]
- Uzarewicz, A.; Wyżlic, I.; Ścianowski, J. Synthesis and reactions of organic-compounds with a nitrogen atom Part 10. Synthesis of allylic 1,2-ditoluene-sulfonamide, 1,2-diamine, hydroxy-1,2-ditoluenesulfonamide and hydroxy-1,2-diamine from (+)-α-phellandrene. Pol. J. Chem. 1995, 69, 681–684. [Google Scholar] [CrossRef]
- Ścianowski, J.; Rafiński, Z.; Wojtczak, A. Syntheses and reactions of new optically active terpene dialkyl diselenides. Eur. J. Org. Chem. 2006, 14, 3216–3225. [Google Scholar] [CrossRef]
- Skowronek, P.; Ścianowski, J.; Gawroński, J. Helicity discrimination in diselenides by chiral substituents—A circular dichroism study. Tetrahedron Asymmetry 2006, 17, 2408–2412. [Google Scholar] [CrossRef]
- Ścianowski, J.; Rafalski, J.; Banach, A.; Czaplewska, J.; Komoszyńska, A. Synthesis and reactions of the optically active selenols derived from monoterpenes. Tetrahedron Asymmetry 2013, 24, 1089–1096. [Google Scholar] [CrossRef]
- Banach, A.; Ścianowski, J.; Uzarewicz-Baig, M.; Wojtczak, A. Terpenyl Selenides: Synthesis and Application in Asymmetric Epoxidation. Eur. J. Org. Chem. 2015, 16, 3477–3485. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Mangiavacchi, F.; Sancineto, L.; Lenardao, E.J.; Ścianowski, J.; Santi, C. An update on “selenium containing compounds from poison to drug candidates: A review on the GPx-like activity”. Curr. Chem. Biol. 2015, 9, 97–112. [Google Scholar] [CrossRef]
- Parnham, J.M.; Sies, H. The early research and development of ebselen. Biochem. Pharm. 2013, 86, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Kil, J.; Lobaris, E.; Spankovich, C.; Griffiths, S.; Antonelli, P.J.; Lynch, E.D.; Le Prell, C.G. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2017, 390, 969–979. [Google Scholar] [CrossRef]
- Singh, N.; Sharpley, N.; Emir, A.L.; Masaki, U.E.; Herzallah, C.; Gluck, M.M.; Sharp, M.A.; Hamer, T.; Vasudevan, C.J.; Coven, S.R.; et al. Effect of the putative lithium mimetic ebselen on brain myo-inositol, sleep, and emotional processing in humans. Neuropsychopharmacology 2016, 41, 1768–1778. [Google Scholar] [CrossRef] [PubMed]
- Orian, L.; Toppo, S. Organochalcogen peroxidase mimetics as potential drugs: A long story of a promise still unfulfilled. Free Rad. Biol. Med. 2014, 66, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wolters, L.P.; Orian, L. Peroxidase Activity of Organic Selenides: Mechanistic Insights from Quantum. Chem. Curr. Org. Chem. 2016, 20, 189–197. [Google Scholar] [CrossRef]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernances, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Rad. Biol. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Aguilar, A.; Romero-Hernández, L.L.; Arenas-González, A.; Merino-Montiel, P.; Montiel-Smith, S.; Meza-Reyes, S.; Vega–Báez, J.L.; Plata, G.B.; Padrón, J.M.; López, Ó.; et al. New selenosteroids as antiproliferative agents. Org. Biomol. Chem. 2017, 15, 5041–5054. [Google Scholar] [CrossRef] [PubMed]
- Stoyanovsky, D.A.; Jiang, J.; Murphy, M.P.; Epperly, M.; Zhang, X.; Li, S.; Greenberger, J.; Kagan, V.; Bayır, H. Design and synthesis of a mitochondria-targeted mimic of glutathione peroxidase, MitoEbselen-2, as a radiation mitigator. ACS Med. Chem. Lett. 2014, 5, 1304–1307. [Google Scholar] [CrossRef] [PubMed]
- Thanna, S.; Goins, C.M.; Knudson, S.E.; Slayden, R.A.; Ronning, D.R.; Sucheck, S.J. Thermal and photoinduced copper-promoted C–Se bond formation: Synthesis of 2-Alkyl-1,2-benzisoselenazol-3(2H)-ones and evaluation against mycobacterium tuberculosis. J. Org. Chem. 2017, 82, 3844–3854. [Google Scholar] [CrossRef] [PubMed]
- Gordhan, H.M.; Patrick, S.L.; Swasy, M.I.; Hackler, A.L.; Anayee, M.; Golden, J.E.; Morris, J.C.; Whitehead, D.C. Evaluation of substituted ebselen derivatives as potential trypanocidal agents. Bioorg. Med. Chem. Lett. 2017, 27, 537–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.P.; Poon, J.; Yan, J.; Lu, X.; Ott, M.K.; Butcher, R.J.; Gates, P.J.; Engman, L. Nitro-, Azo-, and Amino derivatives of ebselen: Synthesis, structure, and cytoprotective effects. J. Org. Chem. 2017, 82, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Welter, A.; Leyck, S.; Etschenberg, E.; Nattermann, A.; Cie GmbH. Benzisoselenazolethiones and Process for the Treatment of Various Diseases in Humans. U.S. Patent 4711961, 8 December 1987. [Google Scholar]
- Shakeel, A.; Altaf, A.A.; Qureshi, A.M.; Badashah, A. Thiourea Derivatives in Drug Design and Medicinal Chemistry: A Short Review. J. Drug Des. Med. Chem. 2016, 2, 10–20. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Kaczor, K.B.; Wójtowicz, A.; Antosiewicz, J.; Janecka, A.; Długosz, A.; Janecki, T.; Ścianowski, J. New glutathione peroxidase mimetics—Insights into antioxidant and cytotoxic activity. Bioorg. Med. Chem. 2017, 25, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, T.; Ertas, E.; Mert, O. Use of Lawesson’s Reagent in Organic Syntheses. Chem. Rev. 2007, 107, 5210–5278. [Google Scholar] [CrossRef] [PubMed]
- Pacuła, A.J.; Ścianowski, J.; Aleksandrzak, K.B. Highly efficient synthesis and antioxidant capacity of N-substituted benzisoselenazol-3(2H)-ones. RSC Adv. 2014, 4, 48959–48962. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Obieziurska, M.; Ścianowski, J.; Kaczor, K.B.; Antosiewicz, J. Synthesis of biologically active diaryl diselenides under water control. Arkivoc 2018, 3, 144–155. [Google Scholar]
- Scheibye, S.; Kristensen, J.; Lawesson, S.O. Studies on organophosphorus compounds—XXVII1: Synthesis of thiono-, thiolo- and dithiolactones. Tetrahedron 1979, 35, 1339–1343. [Google Scholar] [CrossRef]
- Kumakura, F.; Mishra, B.; Priyadarsini, K.I.; Iwaoka, M. A water-soluble cyclic selenide with enhanced glutathione peroxidase-like catalytic activities. Eur. J. Org. Chem. 2010, 440–444. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Kaczor, K.B.; Wójtowicz, A.; Antosiewicz, J.; Janecka, A.; Długosz, A.; Janecki, T.; Ścianowski, J. New chiral ebselen analogues with antioxidant and cytotoxic potential. Molecules 2017, 22, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.; Fisher, A.; Greener, E. The catalytic decomposition of secondary carboxamides by transition-metal complexes. Tetrahedron 1973, 29, 1073–1081. [Google Scholar] [CrossRef]
- Yao, B.; Wang, Q.; Zhu, J. Palladium(II)-catalyzed cyclizative cross-coupling of ortho-Alkynylanilines with ortho-Alkynylbenzamides under aerobic conditions. Angew. Chem. Int. Ed. 2013, 52, 12992–12996. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.M.; Lin, T.; Winchell, H.S.; Medi-Physics, Inc. Amides Useful as Brain Imaging Agents. U.S. Patent 4279887, 21 July 1981. [Google Scholar]
- Pietka-Ottlik, M.; Wojtowicz-Młochowska, H.; Kolodziejczyk, K.; Piasecki, E.; Młochowski, J. New organoselenium compounds active against pathogenic bacteria, fungi and viruses. Chem. Pharm. Bull. 2008, 56, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Welter, A.; Lambert, C.; Dereu, N.; Huther, A.; Etschenberg, E.; Nattermann, A.; Cie GmbH. Benzisoselenazolonyl Derivatives and Processes for the Treatment of Rheumatic Disease. U.S. Patent 4774252, 27 September 1988. [Google Scholar]







| N-Substituted o-Iodobenzothioamides | Yield (%) | |
|---|---|---|
| Method C | Method D | |
| 5a | 38 | 44 |
| 5b | 61 | 73 |
| 5c | 58 | 82 |
| 5d | 48 | 58 |
| 5e | 26 | 57 |
| 5f | 22 | 81 |

| Remaining Dithiotreitol (%) | |||||
|---|---|---|---|---|---|
| Catalyst [0.1 equiv.] | 3 min | 5 min | 15 min | 30 min | 60 min |
| Benzisoselenazolones | |||||
| 6a | 87 | 80 | 65 | 56 | 46 |
| 6b | 77 | 57 | 38 | 26 | 15 |
| 6c | 81 | 59 | 41 | 32 | 29 |
| 6d | 92 | 84 | 81 | 78 | 75 |
| 6e | 77 | 58 | 42 | 28 | 13 |
| 6f | 75 | 69 | 62 | 55 | 44 |
| Benzisoselenazolthiones | |||||
| 7a | 99 | 97 | 96 | 95 | 93 |
| 7b | 43 | 21 | 3 | 2 | 0 |
| 7c | 97 | 97 | 96 | 96 | 95 |
| 7d | 99 | 98 | 98 | 98 | 97 |
| 7e | 40 | 26 | 18 | 17 | 15 |
| 7f | 90 | 84 | 74 | 65 | 47 |
| Ebselen (1) | 84 | 75 | 64 | 58 | 52 |
| Compound | DU-145 | PNT1A |
|---|---|---|
| IC50, µM | ||
| 6a | 30.06 | 20.68 |
| 6b | 30.21 | >60 |
| 6c | 20.76 | >40 |
| 6e | 30.39 | >40 |
| 6f | 5.71 | >40 |
| 7a | 15.33 | 20.29 |
| 7b | 20.36 | 20.77 |
| 7f | 10.7 | 5.63 |
| 40 µM (Cell Viability (%)) | ||
| 6d | 51.429 | 95.669 |
| 7c | 52.161 | 58.060 |
| 7d | 56.829 | 65.027 |
| 7e | 61.177 | 60.74 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obieziurska, M.; Pacuła, A.J.; Juhas, U.; Antosiewicz, J.; Ścianowski, J. The Influence of O/S Exchange on the Biocatalytical Activity of Benzisoselenazol-3(2H)-ones. Catalysts 2018, 8, 493. https://doi.org/10.3390/catal8110493
Obieziurska M, Pacuła AJ, Juhas U, Antosiewicz J, Ścianowski J. The Influence of O/S Exchange on the Biocatalytical Activity of Benzisoselenazol-3(2H)-ones. Catalysts. 2018; 8(11):493. https://doi.org/10.3390/catal8110493
Chicago/Turabian StyleObieziurska, Magdalena, Agata J. Pacuła, Ulana Juhas, Jędrzej Antosiewicz, and Jacek Ścianowski. 2018. "The Influence of O/S Exchange on the Biocatalytical Activity of Benzisoselenazol-3(2H)-ones" Catalysts 8, no. 11: 493. https://doi.org/10.3390/catal8110493
APA StyleObieziurska, M., Pacuła, A. J., Juhas, U., Antosiewicz, J., & Ścianowski, J. (2018). The Influence of O/S Exchange on the Biocatalytical Activity of Benzisoselenazol-3(2H)-ones. Catalysts, 8(11), 493. https://doi.org/10.3390/catal8110493