Promotional Effect of Gold on the WGS Activity of Alumina-Supported Copper-Manganese Mixed Oxides
Abstract
:1. Introduction
2. Results and Discussion
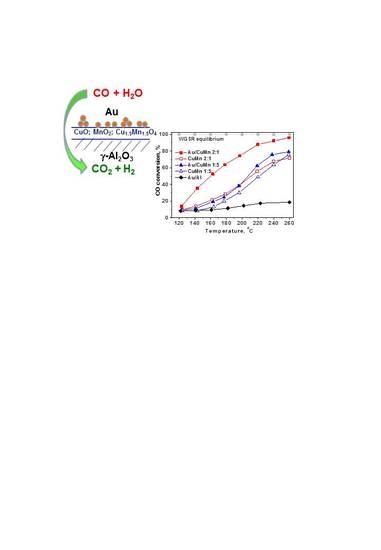
2.1. Catalytic Activity Measurements
2.2. Sample Characterization
2.2.1. Textural Properties
2.2.2. Thermal Analysis (DTA, DTG, and TG)
2.2.3. X-Ray Powder Diffraction (XRD)
2.2.4. High-Resolution Transmission Electron Microscopy (HRTEM)
2.2.5. Electron Paramagnetic Resonance (EPR)
2.2.6. X-Ray Photoelectron Spectroscopy (XPS)
2.2.7. Temperature-Programmed Reduction (TPR)
3. Materials and Methods
3.1. Sample Preparation
3.2. Sample Characterization
3.3. Catalytic Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ratnasamy, C.; Wagner, J.P. Water gas shift catalysis. Catal. Rev. Sci. Eng. 2009, 51, 325–440. [Google Scholar] [CrossRef]
- Pal, D.B.; Chand, R.; Upadhyay, S.N.; Mishra, P.K. Performance of water gas shift reaction catalysts: A review. Renew. Sustain. Energy Rev. 2018, 93, 549–565. [Google Scholar] [CrossRef]
- Tanaka, Y.; Utaka, T.; Kikuchi, R.; Takeguchi, T.; Sasaki, K.; Eguchi, K. Water gas shift reaction for the reformed fuels over Cu/MnO catalysts prepared via spinel-type oxide. J. Catal. 2003, 215, 271–278. [Google Scholar] [CrossRef]
- Tanaka, Y.; Takeguchi, T.; Kikuchi, R.; Eguchi, K. Influence of preparation method and additive for Cu–Mn spinel oxide catalyst on water gas shift reaction of reformed fuels. Appl. Catal. A 2005, 279, 59–66. [Google Scholar] [CrossRef]
- Zhi, K.; Liu, Q.; Zhang, Y.; He, S.; He, R. Effect of precipitator on the texture and activity of copper-manganese mixed oxide catalysts for the water gas shift reaction. J. Fuel Chem. Technol. 2010, 38, 445–451. [Google Scholar] [CrossRef]
- He, R.-X.; Liu, Q.-S.; Zhi, K.-D.; Cui, X.-L. Effect of adding rate of precipitator on the texture and activity of the copper-manganese mixed oxide catalyst for water gas shift reaction. J. Fuel Chem. Technol. 2008, 36, 767–771. [Google Scholar]
- He, R.-X.; Zhi, K.-D.; Wang, B.; Wu, F.; Jiang, H.-Q.; Liu, Q.-S. Influence of copper-manganese salt precursors on catalytic performance of copper-manganese catalysts for water-gas shift reaction. J. Mol. Catal. 2015, 29, 534–544. [Google Scholar]
- Zhi, K.; Liu, Q.; He, R.; Wu, F.; Zhang, Y.; Yang, L. Effect of the ration of Cu to Mn on the activity and stability of copper-manganese mixed-oxides for the water gas shift reaction. Adv. Mater. Res. 2013, 634–638, 518–521. [Google Scholar] [CrossRef]
- Tabakova, T.; Idakiev, V.G.; Avgouropoulos, G.; Papavasiliou, J.; Manzoli, M.; Boccuzzi, F.; Ioannides, T. Highly active copper catalyst for low-temperature water-gas shift reaction prepared via a Cu-Mn spinel oxide precursor. Appl. Catal. A 2013, 413, 184–191. [Google Scholar] [CrossRef]
- Du, X.; Yuan, Z.; Cao, L.; Zhang, C.; Wang, S. Water gas shift reaction over Cu–Mn mixed oxides catalysts: Effects of the third metal. Fuel Proc. Technol. 2008, 89, 131–138. [Google Scholar] [CrossRef]
- He, R.; Wang, D.; Zhi, K.; Wang, B.; Zhong, H.; Jiang, H.; Li, N.; Liu, Q. Cu-Mn catalysts modified by rare earth lanthanum for low temperature water-gas shift reaction. J. Rare Earths 2016, 34, 994–1003. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Oh, H.Y.; Hong, G.H.; Park, J.S.; Kim, J.M. Ordered mesoporous Cu-Mn-Ce ternary metal oxide catalysts for low temperature water-gas shift reaction. Catal. Today 2018, 307, 237–242. [Google Scholar] [CrossRef]
- Andreeva, D.; Idakiev, V.; Tabakova, T.; Andreev, A. Low-Temperature water–gas shift reaction over Au/α-Fe2O3. J. Catal. 1996, 158, 354–355. [Google Scholar] [CrossRef]
- Fu, Q.; Weber, A.; Flytzani-Stephanopuolos, M. Nanostructured Au–CeO2 catalysts for low-temperature water–gas shift. Catal. Lett. 2001, 77, 87–95. [Google Scholar] [CrossRef]
- Sandoval, A.; Gomez-Cortes, A.; Zanella, R.; Diaz, G.; Saniger, J.M. Gold nanoparticles: Support effects for the WGS reaction. J. Mol. Catal. A 2007, 278, 200–208. [Google Scholar] [CrossRef]
- Odabasi, C.; Günay, M.E.; Yildirim, R. Knowledge extraction for water gas shift reaction over noble metal catalysts from publications in the literature between 2002 and 2012. Int. J. Hydrogen Energy 2014, 39, 5733–5746. [Google Scholar] [CrossRef]
- Tao, F.; Ma, Z. Water–gas shift on gold catalysts: Catalyst systems and fundamental studies. Phys. Chem. Chem. Phys. 2013, 15, 15260–15270. [Google Scholar] [CrossRef] [PubMed]
- Reina, T.R.; González Castaño, M.; Palma, S.; Ivanova, S.; Odriozola, J.A. Twenty Years of golden future in the water gas shift reactionin. In Heterogeneous Gold Catalysts and Catalysis; Ma, Z., Dai, S., Eds.; RSC Catal. Series; Royal Society of Chemistry: Cambridge, UK, 2014; Volume 18, pp. 111–140. ISBN 978-1-84973-917-7. [Google Scholar]
- Andreeva, D.; Tabakova, T.; Ilieva, L. Ceria-based gold catalysts: Synthesis, properties and catalytic performance for the WGS and PROX processes. In Catalysis by Ceria and Related Materials, 2nd ed.; Trovarelli, A., Fornasiero, P., Eds.; Imperial College Press: London, UK, 2013; Volume 12, pp. 497–564. ISBN 978-1-84816-963-0. [Google Scholar]
- Reina, T.R.; Ivanova, S.; Idakiev, V.; Delgado, J.J.; Ivanov, I.; Tabakova, T.; Centeno, M.A.; Odriozola, J.A. Impact of Ce-Fe synergism on the catalytic behaviour of Au/CeO2-FeOx/Al2O3 for pure H2 production. Catal. Sci. Technol. 2013, 3, 779–787. [Google Scholar] [CrossRef]
- Reina, T.R.; Ivanova, S.; Delgado, J.J.; Ivanov, I.; Idakiev, V.; Tabakova, T. Viability of Au/CeO2–ZnO/Al2O3 catalysts for pure hydrogen production by the WGSR. ChemCatChem 2014, 5, 1401–1409. [Google Scholar]
- Reina, T.R.; Ivanova, S.; Laguna, O.H.; Centeno, M.A.; Odriozola, J.A. WGS and CO-PrOx reactions using gold promoted copper-ceria catalysts: Bulk CuO-CeO2 vs. CuO-CeO2/Al2O3 with low mixed oxide content. Appl. Catal. B 2016, 197, 62–72. [Google Scholar] [CrossRef]
- Santos, J.L.; Reina, T.R.; Ivanova, S.; Centeno, M.A.; Odriozola, J.A. Gold promoted Cu/ZnO/Al2O3 catalysts prepared from hydrotalcite precursors: Advanced materials for the WGS reaction. Appl. Catal. B 2017, 201, 310–317. [Google Scholar] [CrossRef]
- Ivanov, K.I.; Kolentsova, E.N.; Dimitrov, D.Y. Alumina supported copper-manganese catalysts for combustion of exhaust gases: Effect of preparation method. Int. J. Chem., Nucl. Mater. Metall. Eng. 2015, 4, 548–557. [Google Scholar]
- Roshan, A.C.; Irankhah, A.; Mahmoudizadeh, M.; Arandiyan, H. Single-stage water gas shift reaction over structural modified Cu–Ce catalysts at medium temperatures: Synthesis and catalyst performance. Chem. Eng. Res. Des. 2018, 132, 843–852. [Google Scholar] [CrossRef]
- Kondrat, S.A.; Davies, T.E.; Zu, Z.; Boldrin, P.; Bartley, J.K.; Carley, A.F.; Taylor, S.H.; Rosseinsky, M.J.; Hutchings, G.J. The effect of heat treatment on phase formation of copper manganese oxide: Influence on catalytic activity for ambient temperature carbon monoxide oxidation. J. Catal. 2011, 281, 279–289. [Google Scholar] [CrossRef]
- Pintons, D.G.; Juan, A.; Irigoyen, B. Density functional theory study of water interactions on Mn-doped CeO2(1 1 1) surface. Appl. Surf. Sci. 2014, 313, 784–793. [Google Scholar] [CrossRef]
- Wei, P.; Bieringer, M.; Cranswick, L.; Petric, A. In situ high-temperature X-ray and neutron diffraction of Cu–Mn oxide phases. J. Mater. Sci. 2010, 45, 1056–1064. [Google Scholar] [CrossRef]
- Li, G.; Dimitrijevic, N.M.; Chen, L.; Rajh, T.; Gray, K.A. Role of surface/interfacial Cu2+ sites in the photocatalytic activity of coupled CuO-TiO2 nanocomposites. J. Phys. Chem. C 2008, 112, 19040–19044. [Google Scholar] [CrossRef]
- Dong, L.; Liu, L.; Lv, Y.; Zhu, J.; Wan, H.; Liu, B.; Gao, F.; Wang, X.; Dong, L.; Chen, Y. Surface structure characteristics of CuO/Ti0.5Sn0.5O2 and its activity for CO oxidation. J. Mol. Catal. 2012, 365, 87–94. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Combined steam reforming of methanol over Cu–Mn spinel oxide catalysts. J. Catal. 2007, 251, 7–20. [Google Scholar] [CrossRef]
- Dasireddy, V.D.B.C.; Valand, J.; Likozar, B. PROX reaction of CO in H2/H2O/CO2 Water-Gas Shift (WGS) feedstocks over Cu-Mn/Al2O3 and Cu-Ni/Al2O3 catalysts for fuel cell applications. Renew. Energy 2018, 116, 75–87. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Xia, Q.; Liu, Z.; Li, Z. Catalytic oxidation of toluene over copper and manganese based catalysts: Effect of water vapor. Catal. Commun. 2011, 14, 15–19. [Google Scholar] [CrossRef]
- Morales, M.R.; Barbero, B.P.; Cadús, L.E. Total oxidation of ethanol and propane over Mn-Cu mixed oxide catalysts. Appl. Catal. B 2006, 67, 229–236. [Google Scholar] [CrossRef]
- Manzoli, M.; Chiorino, A.; Vindigni, F.; Boccuzzi, F. Hydrogen interaction with gold nanoparticles and clusters supported on different oxides: A FTIR study. Catal. Today 2012, 181, 62–67. [Google Scholar] [CrossRef]
- Boccuzzi, F.; Chiorino, A.; Manzoli, M.; Andreeva, D.; Tabakova, T. FTIR Study of the Low-Temperature Water–Gas Shift Reaction on Au/Fe2O3 and Au/TiO2 Catalysts. J. Catal. 1999, 188, 176–185. [Google Scholar] [CrossRef]
- Tabakova, T.; Kolentsova, E.; Dimitrov, D.; Ivanov, K.; Manzoli, M.; Venezia, A.M.; Karakirova, Y.; Petrova, P.; Nihtianova, D.; Avdeev, G. CO and VOCs catalytic oxidation over alumina supported Cu–Mn catalysts: Effect of Au or Ag deposition. Top. Catal. 2017, 60, 110–122. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu, and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]










| Sample | SBET (m2/g) | Vtotal (cm3/g) | Dav. (nm) |
|---|---|---|---|
| Al2O3 | 219 | 0.40 | 7.4 |
| CuMn 2:1 | 177 | 0.31 | 7.1 |
| CuMn 1:5 | 181 | 0.32 | 7.2 |
| Au/CuMn 2:1 | 143 | 0.29 | 7.4 |
| Au/CuMn 1:5 | 150 | 0.31 | 6.7 |
| Sample | Weight Loss | |||
|---|---|---|---|---|
| First | Second | Third | Fourth | |
| Onset T, °C (Weight loss, %) | Onset T, °C (Weight loss, %) | Onset T, °C (Weight loss, %) | Onset T, °C (Weight loss, %) | |
| CuMn 2:1 | 54 (5.3) | 160 (12.9) | 182 (5.9) | 303 (6.4) |
| CuMn 1:5 | 46 (5.8) | 148 (17.6) | 190 (2.2) | 331 (2.3) |
| Sample | BE Cu 2p3/2 (eV) | BE Mn 2p3/2 (eV) | BE Au 4f7/2 (eV) | Cu/Mn | Cu/Al | Au/Cu | Au/(Cu + Mn) | Sat/Cu 2p |
|---|---|---|---|---|---|---|---|---|
| Cu-Mn 2:1 | 932.7 933.9 | 642.4 | - | 2.4 | 0.07 | - | - | 0.27 |
| Cu-Mn 1:5 | 932.9 933.6 | 642.2 | - | 1.4 | 0.03 | - | - | 0.18 |
| Au/Cu-Mn 2:1 | 932.7 933.7 | 642.5 | 84.8 | 2.1 | 0.04 | 0.06 | 0.04 | 0.14 |
| Au/Cu-Mn 1:5 | 932.9 933.6 | 642.3 | 84.7 | 1.1 | 0.02 | 0.29 | 0.10 | 0.13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabakova, T.; Ivanov, I.; Karakirova, Y.; Karashanova, D.; Venezia, A.M.; Petrova, P.; Avdeev, G.; Kolentsova, E.; Ivanov, K. Promotional Effect of Gold on the WGS Activity of Alumina-Supported Copper-Manganese Mixed Oxides. Catalysts 2018, 8, 563. https://doi.org/10.3390/catal8110563
Tabakova T, Ivanov I, Karakirova Y, Karashanova D, Venezia AM, Petrova P, Avdeev G, Kolentsova E, Ivanov K. Promotional Effect of Gold on the WGS Activity of Alumina-Supported Copper-Manganese Mixed Oxides. Catalysts. 2018; 8(11):563. https://doi.org/10.3390/catal8110563
Chicago/Turabian StyleTabakova, Tatyana, Ivan Ivanov, Yordanka Karakirova, Daniela Karashanova, Anna Maria Venezia, Petya Petrova, Georgi Avdeev, Elitsa Kolentsova, and Krasimir Ivanov. 2018. "Promotional Effect of Gold on the WGS Activity of Alumina-Supported Copper-Manganese Mixed Oxides" Catalysts 8, no. 11: 563. https://doi.org/10.3390/catal8110563
APA StyleTabakova, T., Ivanov, I., Karakirova, Y., Karashanova, D., Venezia, A. M., Petrova, P., Avdeev, G., Kolentsova, E., & Ivanov, K. (2018). Promotional Effect of Gold on the WGS Activity of Alumina-Supported Copper-Manganese Mixed Oxides. Catalysts, 8(11), 563. https://doi.org/10.3390/catal8110563