Photocatalytic Degradation of Humic Acids Using LaFeO3
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photocatalytic Degradation of HA
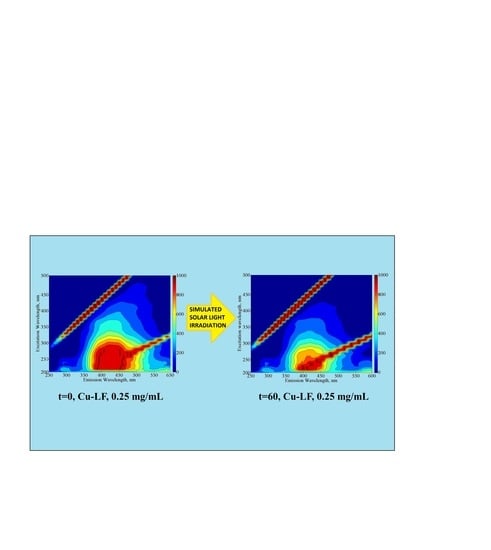
2.2. EEM Fluorescence Properties
3. Materials and Methods
3.1. Materials and Analytical Methodology
3.2. Photocatalytic Degradation Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aiken, G.R.; McKnight, D.M.; Wershaw, R.L.; MacCarthy, P. Humic substances in soil, sediment and water: Geochemistry, isolation and characterization. Geol. J. 1986, 21, 213–221. [Google Scholar]
- Huang, P.M.; Hardie, A.G. Formation mechanisms of humic substances in the environment. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems, 1st ed.; Senesi, N., Xing, B., Huang, P.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 41–109. ISBN 9780470413005. [Google Scholar]
- Birben, N.C.; Paganini, M.C.; Calza, P.; Bekbolet, M. Photocatalytic degradation of humic acid using a novel photocatalyst: Ce-doped ZnO. Photochem. Photobiol. Sci. 2017, 16, 24–30. [Google Scholar] [CrossRef]
- Birben, N.C.; Uyguner-Demirel, C.S.; Kavurmaci, S.S.; Gürkan, Y.Y.; Turkten, N.; Cinar, Z.; Bekbolet, M. Application of Fe-doped TiO2 specimens for the solar photocatalytic degradation of humic acid. Catal. Today 2017, 281, 78–84. [Google Scholar] [CrossRef]
- Birben, N.C.; Uyguner-Demirel, C.S.; Sen-Kavurmaci, S.; Gurkan, Y.Y.; Turkten, N.; Cinar, Z.; Bekbolet, M. Comparative evaluation of anion doped photocatalysts on the mineralization and decolorization of natural organic matter. Catal. Today 2015, 240, 125–131. [Google Scholar] [CrossRef]
- Uyguner, C.S.; Bekbolet, M. Photocatalytic degradation of natural organic matter: Kinetic considerations and light intensity dependence. Int. J. Photoenergy 2004, 6, 73–80. [Google Scholar] [CrossRef]
- Uyguner, C.S.; Bekbolet, M. Aqueous photocatalysis, natural organic matter characterization and removal: A case study of the photacatalytic oxidation of fulvic acid. In Dangerous Pollutants (Xenobiotics) in Urban Water Cycle. NATO Science for Peace and Security Series, 1st ed.; Hlavinek, P., Bonacci, O., Marsalek, J., Mahrikova, I., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 247–257. ISBN 978-1-4020-6800-3. [Google Scholar]
- Uyguner, C.S.; Bekbolet, M. Evaluation of humic acid photocatalytic degradation by UV–vis and fluorescence spectroscopy. Catal. Today 2005, 101, 267–274. [Google Scholar] [CrossRef]
- Uyguner-Demirel, C.S.; Bekbolet, M. Significance of analytical parameters for the understanding of natural organic matter in relation to photocatalytic oxidation. Chemosphere 2011, 84, 1009–1031. [Google Scholar] [CrossRef]
- Anpo, M.; Takeuchi, M. Design and development of second-generation titanium oxide photocatalysts to better our environment—approaches in realizing the use of visible light. Int. J. Photoenergy 2001, 3, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Kanhere, P.; Chen, Z. A review on visible light active perovskite-based photocatalysts. Molecules 2014, 19, 19995–20022. [Google Scholar] [CrossRef]
- Dai, X.P.; Wu, Q.; Li, R.J.; Yu, C.C.; Hao, Z.P. Hydrogen production from a combination of the water−gas shift and redox cycle process of methane partial oxidation via lattice oxygen over LaFeO3 perovskite catalyst. J. Phys. Chem. B 2006, 110, 25856–25862. [Google Scholar] [CrossRef]
- Wei, Z.-X.; Xu, Y.-Q.; Liu, H.-Y.; Hu, C.-W. Preparation and catalytic activities of LaFeO3 and Fe2O3 for hmx thermal decomposition. J. Hazard. Mater. 2009, 165, 1056–1061. [Google Scholar] [CrossRef]
- Caronna, T.; Fontana, F.; Sora, I.N.; Pelosato, R. Chemical synthesis and structural characterization of the substitution compound LaFe1−xCuxO3 (x = 0–0.40). Mater. Chem. Phys. 2009, 116, 645–648. [Google Scholar] [CrossRef]
- Cavalieri, A.; Caronna, T.; Natali Sora, I.; Tulliani, J.M. Electrical characterization of room temperature humidity sensors in La0.8Sr0.2Fe1−xCuxO3 (x = 0, 0.05, 0.10). Ceram. Int. 2012, 38, 2865–2872. [Google Scholar] [CrossRef]
- Mancini, A.; Felice, V.; Natali Sora, I.; Malavasi, L.; Tealdi, C. Chemical compatibility study of melilite-type gallate solid electrolyte with different cathode materials. J. Solid State Chem. 2014, 213, 287–292. [Google Scholar] [CrossRef]
- Natali Sora, I.; Caronna, T.; Fontana, F.; de Julián Fernández, C.; Caneschi, A.; Green, M. Crystal structures and magnetic properties of strontium and copper doped lanthanum ferrites. J. Solid State Chem. 2012, 191, 33–39. [Google Scholar] [CrossRef]
- Natali Sora, I.; Felice, V.; Zurlo, F.; Licoccia, S.; Di Bartolomeo, E. Characterization of tantalum doped lanthanum strontium ferrite as cathode materials for solid oxide fuel cells. J. Alloys Compd. 2015, 648, 154–159. [Google Scholar] [CrossRef]
- Natali Sora, I.; Fontana, F.; Passalacqua, R.; Ampelli, C.; Perathoner, S.; Centi, G.; Parrino, F.; Palmisano, L. Photoelectrochemical properties of doped lanthanum orthoferrites. Electrochimica Acta 2013, 109, 710–715. [Google Scholar] [CrossRef]
- Parrino, F.; García-López, E.; Marcì, G.; Palmisano, L.; Felice, V.; Sora, I.N.; Armelao, L. Cu-substituted lanthanum ferrite perovskites: Preparation, characterization and photocatalytic activity in gas-solid regime under simulated solar light irradiation. J. Alloys Compd. 2016, 682, 686–694. [Google Scholar] [CrossRef]
- Rosa, R.; Ponzoni, C.; Veronesi, P.; Natali Sora, I.; Felice, V.; Leonelli, C. Solution combustion synthesis of La1−xSrxFe1−yCuyO3±w (x = 0, 0.2; y = 0, 0.2) perovskite nanoparticles: Conventional vs. Microwaves ignition. Ceram. Int. 2015, 41, 7803–7810. [Google Scholar] [CrossRef]
- Tulliani, J.-M.; Borgna, M.; Grigioni, I.; Sora, I.N. Microstructural study of aged ferrite powders for sensing layers. Ceram. Int. 2013, 39, 4923–4927. [Google Scholar] [CrossRef]
- Zurlo, F.; Di Bartolomeo, E.; D’Epifanio, A.; Felice, V.; Natali Sora, I.; Tortora, L.; Licoccia, S. La0.8Sr0.2Fe0.8Cu0.2O3−δ as “cobalt-free” cathode for La0.8Sr0.2Ga0.8Mg0.2O3−δ electrolyte. J. Power Sources 2014, 271, 187–194. [Google Scholar] [CrossRef]
- Zurlo, F.; Natali Sora, I.; Felice, V.; Luisetto, I.; D’Ottavi, C.; Licoccia, S.; Di Bartolomeo, E. Copper-doped lanthanum ferrites for symmetric SOFCs. Acta Mater. 2016, 112, 77–83. [Google Scholar] [CrossRef]
- Natali Sora, I.; Fumagalli, D. Fast photocatalytic degradation of pharmaceutical micropollutants and ecotoxicological effects. Environ. Sci. Pollut. R. 2017, 24, 12556–12561. [Google Scholar] [CrossRef]
- Li, F.T.; Liu, Y.; Liu, R.H.; Sun, Z.M.; Zhao, D.S.; Kou, C.G. Preparation of Ca-doped LaFeO3 nanopowders in a reverse microemulsion and their visible light photocatalytic activity. Mater. Lett. 2010, 64, 223–225. [Google Scholar] [CrossRef]
- Li, S.; Jing, L.; Fu, W.; Yang, L.; Xin, B.; Fu, H. Photoinduced charge property of nanosized perovskite-type LaFeO3 and its relationships with photocatalytic activity under visible irradiation. Mater. Res. Bull. 2007, 42, 203–212. [Google Scholar] [CrossRef]
- Parida, K.M.; Reddy, K.H.; Martha, S.; Das, D.P.; Biswal, N. Fabrication of nanocrystalline LaFeO3: An efficient sol–gel auto-combustion assisted visible light responsive photocatalyst for water decomposition. Int. J. Hydrog. Energy 2010, 35, 12161–12168. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Girija, K.; Hebalkar, N.Y.; Mangalaraj, D.; Viswanathan, C.; Ponpandian, N. Shape evolution of perovskite LaFeO3 nanostructures: A systematic investigation of growth mechanism, properties and morphology dependent photocatalytic activities. RSC Adv. 2013, 3, 7549–7561. [Google Scholar] [CrossRef]
- Jing, L.; Qu, Y.; Su, H.; Yao, C.; Fu, H. Synthesis of high-activity TiO2-based photocatalysts by compounding a small amount of porous nanosized LaFeO3 and the activity-enhanced mechanisms. J. Phys. Chem. C 2011, 115, 12375–12380. [Google Scholar] [CrossRef]
- Dhinesh Kumar, R.; Thangappan, R.; Jayavel, R. Synthesis and characterization of LaFeO3/TiO2 nanocomposites for visible light photocatalytic activity. J. Phys. Chem. Solids 2017, 101, 25–33. [Google Scholar] [CrossRef]
- Gao, K.; Li, S. Multi-modal TiO2–LaFeO3 composite films with high photocatalytic activity and hydrophilicity. Appl. Surf. Sci. 2012, 258, 6460–6464. [Google Scholar] [CrossRef]
- Jamali, S.S.; Singh, D.; Tavakkoli, H.; Kaveh, F.; Tabari, T. Microwave-assisted synthesis of nanostructured perovskite-type oxide with efficient photocatalytic activity against organic reactants in gaseous and aqueous phases. Mater. Sci. Semicond. Process. 2017, 64, 47–54. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, H.; Li, R.; Wang, X. Enhanced photocatalytic performance and mechanism of Ag-decorated LaFeO3 nanoparticles. J. Sol-Gel Sci. Technol. 2017, 82, 509–518. [Google Scholar] [CrossRef]
- Uyguner, C.S.; Bekbolet, M. Implementation of spectroscopic parameters for practical monitoring of natural organic matter. Desalination 2005, 176, 47–55. [Google Scholar] [CrossRef]
- Sen-Kavurmaci, S.; Birben, N.C.; Tomruk, A.; Bekbolet, M. Characterization of organic matter in natural waters by EEM fluorescence properties. Desalin. Water Treat. 2016, 57, 2428–2436. [Google Scholar] [CrossRef]
- Uyguner-Demirel, C.S.; Birben, C.; Bekbolet, M. Key role of common anions on the photocatalytic degradation profiles of the molecular size fractions of humic acids. Catal. Today 2013, 209, 122–126. [Google Scholar] [CrossRef]
- Rusevova, K.; Köferstein, R.; Rosell, M.; Richnow, H.H.; Kopinke, F.-D.; Georgi, A. LaFeO3 and BiFeO3 perovskites as nanocatalysts for contaminant degradation in heterogeneous Fenton-like reactions. Chem. Eng. J. 2014, 239, 322–331. [Google Scholar] [CrossRef]
- Uyguner, C.S.; Bekbolet, M. A review on the photocatalytic degradation of humic substances. In Control of Disinfection By-Products in Drinking Water Systems; Nikolau, A., Selcuk, H., Rizzo, L., Eds.; NOVA Science Publishers Inc.: New York, NY, USA, 2007; Chapter 7.4; pp. 419–446. [Google Scholar]
- Bekbolet, M.; Sen-Kavurmaci, S. The effect of photocatalytic oxidation on molecular size distribution profiles of humic acid. Photochem. Photobiol. Sci. 2015, 14, 576–582. [Google Scholar] [CrossRef]
- Kerc, A.; Bekbolet, M.; Saatci, A.M. Effects of oxidative treatment techniques on molecular size distribution of humic acids. Water Sci. Technol. 2004, 49, 7–12. [Google Scholar] [CrossRef]
- Abbt-Braun, G.; Frimmel, F.H. Dissolved organic matter (DOM) in natural environments. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems, 1st ed.; Senesi, N., Xing, B., Huang, P.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 367–406. ISBN 9780470413005. [Google Scholar]




| Photocatalyst Specimen | Color436 | UV365 | UV280 | UV254 | DOC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rate m−1/min | t½ min | Rate m−1/min | t½ min | Rate m−1/min | t½ min | Rate m−1/min | t½ min | Rate m−1/min | t½ min | |
| LF | 2.2 × 10−4 | 81 | 5.4 × 10−4 | 64 | 1.4 × 10−3 | 63 | 1.7 × 10−3 | 60 | 3.0 × 10−2 | 60 |
| Cu-LF | 1.7 × 10−3 | 34 | 2.4 × 10−3 | 33 | 2.8 × 10−3 | 35 | 5.1 × 10−3 | 36 | 3.1 × 10−2 | 77 |
| Sample | Removal % | CbUV254 L/mg m | Fluorescence Index | ||||
|---|---|---|---|---|---|---|---|
| Color436 | UV365 | UV280 | UV254 | DOC | |||
| 30 DaHA | - | - | - | - | - | 6.89 | 1.04 |
| LF, 0.25 mg/mL | 40.8 | 47.8 | 49.7 | 51.0 | 54.5 | 7.44 | 1.12 |
| LF, 0.50 mg/mL | 68.2 | 70.9 | 70.8 | 71.8 | 52.7 | 4.11 | 1.18 |
| Cu-LF, 0.25 mg/mL | 70.2 | 72.5 | 70.0 | 68.4 | 56.3 | 3.03 | 1.36 |
| Cu-LF, 0.50 mg/mL | 47.9 | 58.9 | 63.4 | 63.8 | 37.5 | 1.73 | 1.17 |
| 100 kDaHA | - | - | - | - | - | 9.47 | 0.992 |
| LF, 0.25 mg/mL | 32.7 | 31.0 | 32.0 | 31.6 | 26.2 | 12.4 | 1.06 |
| LF, 0.50 mg/mL | 64.6 | 68.4 | 66.8 | 67.6 | 43.8 | 7.70 | 1.10 |
| Cu-LF, 0.25 mg/mL | 10.8 | 22.7 | 30.0 | 30.0 | 45.3 | 12.1 | 1.08 |
| Cu-LF, 0.50 mg/mL | 14.6 | 28.1 | 37.4 | 38.5 | 38.2 | 9.41 | 1.11 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turkten, N.; Natali Sora, I.; Tomruk, A.; Bekbolet, M. Photocatalytic Degradation of Humic Acids Using LaFeO3. Catalysts 2018, 8, 630. https://doi.org/10.3390/catal8120630
Turkten N, Natali Sora I, Tomruk A, Bekbolet M. Photocatalytic Degradation of Humic Acids Using LaFeO3. Catalysts. 2018; 8(12):630. https://doi.org/10.3390/catal8120630
Chicago/Turabian StyleTurkten, Nazli, Isabella Natali Sora, Ayse Tomruk, and Miray Bekbolet. 2018. "Photocatalytic Degradation of Humic Acids Using LaFeO3" Catalysts 8, no. 12: 630. https://doi.org/10.3390/catal8120630
APA StyleTurkten, N., Natali Sora, I., Tomruk, A., & Bekbolet, M. (2018). Photocatalytic Degradation of Humic Acids Using LaFeO3. Catalysts, 8(12), 630. https://doi.org/10.3390/catal8120630