Catalytic Activities of Noble Metal Phosphides for Hydrogenation and Hydrodesulfurization Reactions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrogenation of Biphenyl
2.2. Hydrodesulfization of Dibenzothiophene
2.2.1. Activities of NM-P Catalysts
2.2.2. XRD Patterns of NM-P Catalysts after the HDS of DBT
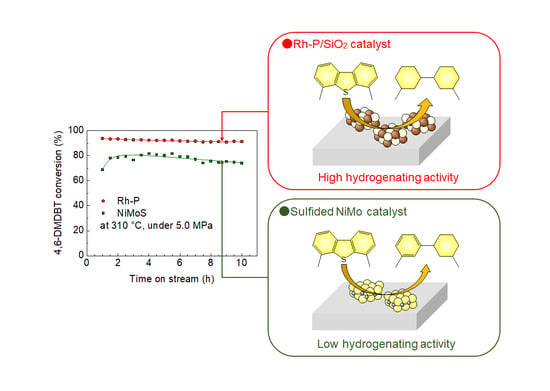
2.3. Hydrodesulfization of 4,6-Dimethyldibenzothiophene
2.3.1. Effect of Reaction Temperature on Catalytic Activity
2.3.2. Effect of Reaction Pressure on Catalytic Activity
2.3.3. XRD Patterns of Rh-P Catalyst after the HDS of 4,6-DMDBT
2.4. Potential of Rh2P as a New HDS Catalyst
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Catalytic Reactions
3.3. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Okamoto, Y. A novel preparation-characterization technique of hydrodesulfurization catalysts for cleaner fuels. Catal. Today 2008, 132, 9–17. [Google Scholar] [CrossRef]
- Oyama, S.T.; Gott, T.; Zhao, H.; Lee, Y.K. Transition metal phosphide hydroprocessing catalysts: A review. Catal. Today 2009, 143, 94–107. [Google Scholar] [CrossRef]
- Prins, R.; Bussell, M.E. Metal Phosphides: Preparation, Characterization and Catalytic Reactivity. Catal. Lett. 2012, 142, 1413–1436. [Google Scholar] [CrossRef]
- Okamoto, Y. Novel Molecular Approaches to the Structure Activity Relationships and Unique Characterizations of CoMo Sulfide Hydrodesulfurization Catalysts for the Production of Ultraclean Fuels. Bull. Chem. Soc. Jpn. 2014, 87, 20–58. [Google Scholar] [CrossRef]
- Shu, Y.; Lee, Y.K.; Oyama, S.T. Structure-sensitivity of hydrodesulfurization of 4,6-dimethyldibenzothiophene over silica-supported nickel phosphide catalysts. J. Catal. 2005, 236, 112–121. [Google Scholar] [CrossRef]
- Niquille-Röthlisberger, A.; Prins, R. Hydrodesulfurization of 4,6-dimethyldibenzothiophene and dibenzothiophene over alumina-supported Pt, Pd, and Pt-Pd catalysts. J. Catal. 2006, 242, 207–216. [Google Scholar] [CrossRef]
- Vít, Z.; Gulková, D.; Kaluža, L.; Kupčík, J. Pd-Pt catalysts on mesoporous SiO2-Al2O3 with superior activity for HDS of 4,6-dimethyldibenzothiophene: Effect of metal loading and support composition. Appl. Catal. B Environ. 2015, 179, 44–53. [Google Scholar] [CrossRef]
- Robinson, W.R.A.M.; van Gestel, J.N.M.; Koranyi, T.I.; Eijsbouts, S.; van der Kraan, A.M.; van Veen, J.A.R.; de Beer, V.H.J. Phosphorus Promotion of Ni(Co)-Containing Mo-Free Catalysts in Quinoline Hydrodenitrogenation. J. Catal. 1996, 161, 539–550. [Google Scholar] [CrossRef]
- Stinner, C.; Tang, Z.; Haouas, M.; Weber, T.; Prins, R. Preparation and 31P NMR Characterization of Nickel Phosphides on Silica. J. Catal. 2002, 208, 456–466. [Google Scholar] [CrossRef]
- Wang, A.; Ruan, L.; Teng, Y.; Li, X.; Lu, M.; Rena, J.; Wanga, Y.; Hu, Y. Hydrodesulfurization of dibenzothiophene over siliceous MCM-41-supported nickel phosphide catalysts. J. Catal. 2005, 229, 314–321. [Google Scholar] [CrossRef]
- Lee, Y.K.; Oyama, S.T. Bifunctional nature of a SiO2-supported Ni2P catalyst for hydrotreating: EXAFS and FTIR studies. J. Catal. 2006, 239, 376–389. [Google Scholar] [CrossRef]
- Wang, R.; Smith, K.J. Hydrodesulfurization of 4,6-dimethyldibenzothiophene over high surface area metal phosphides. Appl. Catal. A Gen. 2009, 361, 18–25. [Google Scholar] [CrossRef]
- Da Silva, V.T.; Sousa, L.A.; Amorim, R.M.; Andrini, L.; Figueroa, S.J.A.; Requejo, F.G.; Vicentini, F.C. Lowering the synthesis temperature of Ni2P/SiO2 by palladium addition. J. Catal. 2011, 279, 88–102. [Google Scholar] [CrossRef]
- Oyama, S.T.; Zhao, H.; Freund, H.J.; Asakura, K.; Włodarczyk, R.; Sierka, M. Unprecedented selectivity to the direct desulfurization (DDS) pathway in a highly active FeNi bimetallic phosphide catalyst. J. Catal. 2012, 285, 1–5. [Google Scholar] [CrossRef]
- Lee, Y.K.; Oyama, S.T. Sulfur resistant nature of Ni2P catalyst in deep hydrodesulfurization. Appl. Catal. A Gen. 2017, 548, 103–113. [Google Scholar] [CrossRef]
- Kanda, Y.; Temma, C.; Nakata, K.; Kobayashi, T.; Sugioka, M.; Uemichi, Y. Preparation and performance of noble metal phosphides supported on silica as new hydrodesulfurization catalysts. Appl. Catal. A Gen. 2010, 386, 171–178. [Google Scholar] [CrossRef]
- Kanda, Y.; Temma, C.; Sawada, A.; Sugioka, M.; Uemichi, Y. Formation of active sites and hydrodesulfurization activity of rhodium phosphide catalyst: Effect of reduction temperature and phosphorus loading. Appl. Catal. A Gen. 2014, 457, 410–419. [Google Scholar] [CrossRef]
- Kanda, Y.; Uemichi, Y. Noble Metal Phosphides as New Hydrotreating Catalysts: Highly Active Rhodium Phosphide Catalyst. J. Jpn. Petrol. Inst. 2015, 58, 20–32. [Google Scholar] [CrossRef]
- Kanda, Y.; Matsukura, Y.; Sawada, A.; Sugioka, M.; Uemichi, Y. Low-temperature synthesis of rhodium phosphide on alumina and investigation of its catalytic activity toward the hydrodesulfurization of thiophene. Appl. Catal. A Gen. 2016, 515, 25–31. [Google Scholar] [CrossRef]
- Hayes, J.R.; Bowker, R.H.; Gaudette, A.F.; Smith, M.C.; Moak, C.E.; Nam, C.Y.; Pratum, T.K.; Bussell, M.E. Hydrodesulfurization properties of rhodium phosphide: Comparison with rhodium metal and sulfide catalysts. J. Catal. 2010, 276, 249–258. [Google Scholar] [CrossRef]
- Bowker, R.H.; Smith, M.C.; Carrillo, B.A.; Bussell, M.E. Synthesis and Hydrodesulfurization Properties of Noble Metal Phosphides: Ruthenium and Palladium. Top. Catal. 2012, 55, 999–1009. [Google Scholar] [CrossRef]
- Belykh, L.B.; Skripov, N.I.; Belonogova, L.N.; Umanets, V.A.; Stepanova, T.P.; Schmidt, F.K. Nature of the modifying action of white phosphorus on the properties of nanosized hydrogenation catalysts based on bis(dibenzylideneacetone)palladium(0). Kinet. Catal. 2011, 52, 702–710. [Google Scholar] [CrossRef]
- Kanda, Y.; Seino, A.; Kobayashi, T.; Uemichi, Y.; Sugioka, M. Hydrodesulfurization of Benzothiophene over Noble Metal Catalysts Supported on Mesoporous Silica MCM-41. J. Jpn. Petrol. Inst. 2009, 52, 42–50. [Google Scholar] [CrossRef]
- Neurock, M.; van Santen, R.A. Theory of Carbon-Sulfur Bond Activation by Small Metal Sulfide Particles. J. Am. Chem. Soc. 1994, 116, 4427–4439. [Google Scholar] [CrossRef]
- International Center for Diffraction Data. PDF-2 2014 (Database); International Center for Diffraction Data: Newtown, PA, USA, 2014. [Google Scholar]
- Okamoto, Y.; Kato, A.; Usman Rinaldi, N.; Fujikawa, T.; Koshika, H.; Hiromitsu, I.; Kubota, T. Effect of sulfidation temperature on the intrinsic activity of Co–MoS2 and Co–WS2 hydrodesulfurization catalysts. J. Catal. 2009, 265, 216–228. [Google Scholar] [CrossRef]
- Wu, H.; Duan, A.; Zhao, Z.; Qi, D.; Li, J.; Liu, B.; Jiang, G.; Liu, J.; Wei, Y.; Zhang, X. Preparation of NiMo/KIT-6 hydrodesulfurization catalysts with tunable sulfidation and dispersion degrees of active phase by addition of citric acid as chelating agent. Fuel 2014, 130, 203–210. [Google Scholar] [CrossRef]
- Dufresne, P. Hydroprocessing catalysts regeneration and recycling. Appl. Catal. A Gen. 2006, 322, 67–75. [Google Scholar] [CrossRef]
- PGM Market Report. Available online: http://www.platinum.matthey.com/documents/new-item/pgm%20market%20 reports/pgm_market_report_february_2018.pdf (accessed on 23 March 2018).











| Catalyst | Yield (%) | |
|---|---|---|
| CHB | BCH | |
| Rh-P | 74.3 | 13.3 |
| Pd-P | 6.7 | 0.0 |
| Ru-P | 2.0 | 0.0 |
| NiMoS | 26.7 | 0.8 |
| Catalyst | Temperature | Conversion | Selectivity (%) | |||
|---|---|---|---|---|---|---|
| (°C) | (%) | BP | CHB | BCH | HDBTs | |
| Rh-P | 210 | 29.1 | 22.3 | 33.9 | 5.9 | 37.8 |
| 230 | 43.7 | 20.0 | 40.0 | 11.7 | 28.3 | |
| 250 | 76.3 | 11.9 | 39.2 | 18.8 | 30.2 | |
| 270 | 95.2 | 12.4 | 44.2 | 35.8 | 7.7 | |
| Pd-P | 210 | 8.7 | 13.4 | 13.7 | 0.0 | 73.0 |
| 230 | 16.2 | 12.7 | 19.6 | 6.4 | 61.3 | |
| 250 | 26.4 | 19.5 | 31.8 | 7.5 | 41.1 | |
| 270 | 43.9 | 23.8 | 36.4 | 10.7 | 29.0 | |
| Ru-P | 210 | 1.0 | 100.0 | n.d | n.d | n.d |
| 230 | 2.1 | 76.1 | n.d | n.d | 23.9 | |
| 250 | 6.7 | 39.5 | 13.2 | n.d | 47.3 | |
| 270 | 15.1 | 49.3 | 20.3 | n.d | 30.4 | |
| NiMoS | 210 | 14.0 | 62.3 | 9.7 | n.d | 28.0 |
| 230 | 62.5 | 66.3 | 28.3 | 0.8 | 4.6 | |
| 250 | 94.2 | 79.4 | 19.3 | 0.4 | 0.8 | |
| 270 | 98.8 | 74.1 | 21.7 | 0.5 | 3.6 | |
| Catalyst | Temperature | Conversion | Selectivity (%) | |||
|---|---|---|---|---|---|---|
| (°C) | (%) | DMBP | MCHTs | DMBCHs | HDMDBTs | |
| Rh-P | 250 | 28.7 | n.d | 7.1 | 9.6 | 50.5 |
| 270 | 61.0 | 1.5 | 16.9 | 21.6 | 29.6 | |
| 290 | 63.5 | 3.0 | 19.7 | 35.2 | 26.8 | |
| 310 | 71.7 | 4.3 | 28.3 | 52.2 | 11.6 | |
| NiMoS | 250 | 29.2 | 3.5 | 57.3 | 9.8 | 17.6 |
| 270 | 75.0 | 6.9 | 65.9 | 15.7 | 6.5 | |
| 290 | 73.3 | 9.3 | 62.6 | 18.6 | 4.4 | |
| 310 | 66.6 | 17.7 | 51.3 | 16.8 | 4.3 | |
| Catalyst | Pressure | Conversion | Selectivity (%) | |||
|---|---|---|---|---|---|---|
| (MPa) | (%) | DMBP | MCHTs | DMBCHs | HDMDBTs | |
| Rh-P | 3.0 | 64.5 | 11.6 | 41.0 | 33.9 | 11.6 |
| 4.0 | 71.7 | 4.3 | 28.3 | 52.2 | 11.6 | |
| 5.0 | 91.3 | 1.9 | 23.1 | 63.3 | 5.9 | |
| NiMoS | 3.0 | 61.2 | 21.1 | 53.3 | 14.9 | 4.6 |
| 4.0 | 66.6 | 17.7 | 51.3 | 16.8 | 4.3 | |
| 5.0 | 74.1 | 11.0 | 60.9 | 18.8 | 2.0 | |
| Reaction Condition | Crystallite Size of Rh2P (nm) | |
|---|---|---|
| Temperature (°C) | Pressure (MPa) | |
| 250 | 4.0 | 7.3 |
| 270 | 4.0 | 7.6 |
| 290 | 4.0 | 7.9 |
| 310 | 3.0 | 7.0 |
| 310 | 4.0 | 7.7 |
| 310 | 5.0 | 7.2 |
| Catalyst | Optimal Reduction Temperature for HDS of Thiophene (°C) | NMXPY Phase Observed by XRD | Reference |
|---|---|---|---|
| Rh-P | 550 | Rh2P, Rh (small peak) | [16,17] |
| Pd-P | 500 | Pd6P, Pd9P2 (Pd4.8P), Pd3P, Pd5P2 | [16,18] |
| Ru-P | 650 | Ru2P, Ru (small peak) | [16,18] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanda, Y.; Kawanishi, K.; Tsujino, T.; Al-otaibi, A.M.; Uemichi, Y. Catalytic Activities of Noble Metal Phosphides for Hydrogenation and Hydrodesulfurization Reactions. Catalysts 2018, 8, 160. https://doi.org/10.3390/catal8040160
Kanda Y, Kawanishi K, Tsujino T, Al-otaibi AM, Uemichi Y. Catalytic Activities of Noble Metal Phosphides for Hydrogenation and Hydrodesulfurization Reactions. Catalysts. 2018; 8(4):160. https://doi.org/10.3390/catal8040160
Chicago/Turabian StyleKanda, Yasuharu, Kota Kawanishi, Taiki Tsujino, Ahmad MFM Al-otaibi, and Yoshio Uemichi. 2018. "Catalytic Activities of Noble Metal Phosphides for Hydrogenation and Hydrodesulfurization Reactions" Catalysts 8, no. 4: 160. https://doi.org/10.3390/catal8040160
APA StyleKanda, Y., Kawanishi, K., Tsujino, T., Al-otaibi, A. M., & Uemichi, Y. (2018). Catalytic Activities of Noble Metal Phosphides for Hydrogenation and Hydrodesulfurization Reactions. Catalysts, 8(4), 160. https://doi.org/10.3390/catal8040160