Low-Temperature Activity and PdO-PdOx Transition in Methane Combustion by a PdO-PdOx/γ-Al2O3 Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Preparation
2.2. Catalyst Characterization
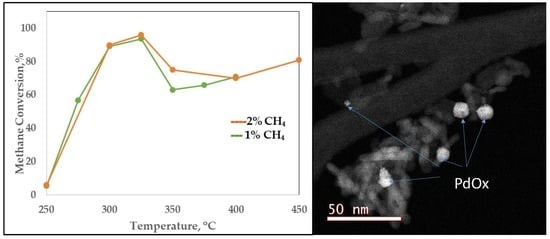
2.2.1. Scanning Transmission Electron Microscopy (STEM) and EDS Elementary Mapping
2.2.2. X-ray Photoelectron Spectroscopy (XPS)
2.2.3. X-ray Powder Diffraction
2.2.4. Physisorption and Chemisorption
2.3. Activity Studies
2.4. Active Phase of the Catalyst and Proposed Mechanism
- (i)
- The active phase of the catalyst is Pd native oxide (PdOx); during the catalyst preparation and calcination process, the oxide ions from the support, γ-Al2O3, migrate to PdO to form PdOx. This mechanism supports the presence of both PdO and PdOx in the catalyst before reaction.
- (ii)
- During the reaction at lower temperatures below 325 °C, oxide ions from PdOx migrate to react with methane; PdOx forms PdO; and oxide ions from the support (γ-Al2O3) migrate to PdO to form PdOx. This process maintains the surface composition of both PdO and PdOx; the catalytic activity remains more or less steady since the active phase of PdOx does not deplete.
- (iii)
- At a higher temperature when catalytic activity decreases in the temperature range 325–450 °C, oxide ions from PdOx migrate to react with methane; and PdOx forms PdO. However, due to unknown reasons (may be due to deactivation by steam) the support does not provide oxide ions to PdO and thereby the number of active sites of PdOx decreases and hence activity decreases. This hypothesis supports the correlation between decrease in catalytic activity and depletion of PdOx at temperatures in the range 325–400 °C as shown by XPS.
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
3.3. Activity Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farrauto, R.J. Low-temperature oxidation of methane. Science 2012, 337, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Gélin, P.; Primet, M. Complete oxidation of methane at low temperature over noble metal based catalysts: A review. Appl. Catal. B Environ. 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Simplício, L.M.T.; Brandão, S.T.; Sales, E.A.; Lietti, L.; Bozon-Verduraz, F. Methane combustion over pdo-alumina catalysts: The effect of palladium precursors. Appl. Catal. B Environ. 2006, 63, 9–14. [Google Scholar] [CrossRef]
- Yoshida, H.; Nakajima, T.; Yazawa, Y.; Hattori, T. Support effect on methane combustion over palladium catalysts. Appl. Catal. B Environ. 2007, 71, 70–79. [Google Scholar] [CrossRef]
- Gholami, R.; Smith, K.J. Activity of pdo/SiO2 catalysts for Ch4 oxidation following thermal treatments. Appl. Catal. B Environ. 2015, 168–169, 156–163. [Google Scholar] [CrossRef]
- Goodman, E.D.; Dai, S.; Yang, A.-C.; Wrasman, C.J.; Gallo, A.; Bare, S.R.; Hoffman, A.S.; Jaramillo, T.F.; Graham, G.W.; Pan, X.; et al. Uniform pt/pd bimetallic nanocrystals demonstrate platinum effect on palladium methane combustion activity and stability. ACS Catal. 2017, 7, 4372–4380. [Google Scholar] [CrossRef]
- Stefanov, P.; Todorova, S.; Naydenov, A.; Tzaneva, B.; Kolev, H.; Atanasova, G.; Stoyanova, D.; Karakirova, Y.; Aleksieva, K. On the development of active and stable pd–co/γ-Al2O3 catalyst for complete oxidation of methane. Chem. Eng. J. 2015, 266, 329–338. [Google Scholar] [CrossRef]
- Ercolino, G.; Stelmachowski, P.; Specchia, S. Catalytic performance of pd/Co3O4 on sic and ZrO2 open cell foams for process intensification of methane combustion in lean conditions. Ind. Eng. Chem. Res. 2017, 56, 6625–6636. [Google Scholar] [CrossRef]
- Cargnello, M.; Delgado Jaen, J.J.; Hernandez Garrido, J.C.; Bakhmutsky, K.; Montini, T.; Calvino Gamez, J.J.; Gorte, R.J.; Fornasiero, P. Exceptional activity for methane combustion over modular pd@CeO2 subunits on functionalized Al2O3. Science 2012, 337, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Khader, M.; Al-Marri, M.; Ali, S.; Abdelmoneim, A. Active and stable methane oxidation nano-catalyst with highly-ionized palladium species prepared by solution combustion synthesis. Catalysts 2018, 8, 66. [Google Scholar] [CrossRef]
- Xiao, C.; Yang, Y.; Meng, D.; Dong, L.; Luo, L.; Tan, Z. Stable and active monolithic palladium catalyst for catalytic oxidation of methane using nanozeolite silicalite-1 coating on cordierite. Appl. Catal. A Gen. 2017, 531, 197–202. [Google Scholar] [CrossRef]
- Li, M.; Gui, P.; Zheng, L.; Li, J.; Xue, G.; Liang, J. Active component migration and catalytic properties of nitrogen modified composite catalytic materials. Catalysts 2018, 8, 125. [Google Scholar] [CrossRef]
- Zhang, X.; Long, E.; Li, Y.; Zhang, L.; Guo, J.; Gong, M.; Chen, Y. The effect of CeO2 and bao on pd catalysts used for lean-burn natural gas vehicles. J. Mol. Catal. A Chem. 2009, 308, 73–78. [Google Scholar] [CrossRef]
- Venezia, A.M.; La Parola, V.; Liotta, L.F. Structural and surface properties of heterogeneous catalysts: Nature of the oxide carrier and supported particle size effects. Catal. Today 2017, 285, 114–124. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Hobson, M.C.; Kennelly, T.; Waterman, E.M. Catalytic chemistry of supported palladium for combustion of methane. Appl. Catal. A Gen. 1992, 81, 227–237. [Google Scholar] [CrossRef]
- Chin, Y.-H.; Buda, C.; Neurock, M.; Iglesia, E. Consequences of metal–oxide interconversion for c–h bond activation during CH4 reactions on pd catalysts. J. Am. Chem. Soc. 2013, 135, 15425–15442. [Google Scholar] [CrossRef] [PubMed]
- Gholami, R.; Alyani, M.; Smith, K. Deactivation of pd catalysts by water during low temperature methane oxidation relevant to natural gas vehicle converters. Catalysts 2015, 5, 561. [Google Scholar] [CrossRef]
- Sadokhina, N.; Ghasempour, F.; Auvray, X.; Smedler, G.; Nylén, U.; Olofsson, M.; Olsson, L. An experimental and kinetic modelling study for methane oxidation over pd-based catalyst: Inhibition by water. Catal. Lett. 2017, 147, 2360–2371. [Google Scholar] [CrossRef]
- Bychkov, V.Y.; Tyulenin, Y.P.; Gorenberg, A.Y.; Sokolov, S.; Korchak, V.N. Evolution of pd catalyst structure and activity during catalytic oxidation of methane and ethane. Appl. Catal. A Gen. 2014, 485, 1–9. [Google Scholar] [CrossRef]
- Miller, J.B.; Malatpure, M. Pd catalysts for total oxidation of methane: Support effects. Appl. Catal. A Gen. 2015, 495, 54–62. [Google Scholar] [CrossRef]
- Kinnunen, N.M.; Hirvi, J.T.; Venäläinen, T.; Suvanto, M.; Pakkanen, T.A. Procedure to tailor activity of methane combustion catalyst: Relation between pd/pdox active sites and methane oxidation activity. Appl. Catal. A Gen. 2011, 397, 54–61. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Pfefferle, L.D. Combustion of methane over palladium-based catalysts: Support interactions. J. Phys. Chem. C 2012, 116, 8571–8578. [Google Scholar] [CrossRef]
- Zhao, M.; Florin, N.H.; Harris, A.T. Mesoporous supported cobalt catalysts for enhanced hydrogen production during cellulose decomposition. Appl. Catal. B Environ. 2010, 97, 142–150. [Google Scholar] [CrossRef]
- Vasilev, M.P.; Abiev, R.S. Dispersion of carbon nanotubes clusters in pulsating and vortex in-line apparatuses. Chem. Eng. Sci. 2017, 171, 204–217. [Google Scholar] [CrossRef]
- Crist, B.V. Handbook of Monochromatic Xps Spectra. The Elements of Native Oxides; Wiley-VCH: Weinheim, Germany, 2000; ISBN 0-471-49265-5. [Google Scholar]
- Xiong, H.; Wiebenga, M.H.; Carrillo, C.; Gaudet, J.R.; Pham, H.N.; Kunwar, D.; Oh, S.H.; Qi, G.; Kim, C.H.; Datye, A.K. Design considerations for low-temperature hydrocarbon oxidation reactions on pd based catalysts. Appl. Catal. B Environ. 2018, 236, 436–444. [Google Scholar] [CrossRef]
- Huang, F.; Chen, J.; Hu, W.; Li, G.; Wu, Y.; Yuan, S.; Zhong, L.; Chen, Y. Pd or pdo: Catalytic active site of methane oxidation operated close to stoichiometric air-to-fuel for natural gas vehicles. Appl. Catal. B Environ. 2017, 219, 73–81. [Google Scholar] [CrossRef]
- Ercolino, G.; Karimi, S.; Stelmachowski, P.; Specchia, S. Catalytic combustion of residual methane on alumina monoliths and open cell foams coated with Pd/Co3O4. Chem. Eng. J. 2017, 326, 339–349. [Google Scholar] [CrossRef]
- Ercolino, G.; Stelmachowski, P.; Grzybek, G.; Kotarba, A.; Specchia, S. Optimization of pd catalysts supported on Co3O4 for low-temperature lean combustion of residual methane. Appl. Catal. B Environ. 2017, 206, 712–725. [Google Scholar] [CrossRef]
- Duggan, J.N.; Bozack, M.J.; Roberts, C.B. The synthesis and arrested oxidation of amorphous cobalt nanoparticles using dmso as a functional solvent. J. Nanopart. Res. 2013, 15, 2089. [Google Scholar] [CrossRef]
- Sulmonetti, T.P.; Pang, S.H.; Claure, M.T.; Lee, S.; Cullen, D.A.; Agrawal, P.K.; Jones, C.W. Vapor phase hydrogenation of furfural over nickel mixed metal oxide catalysts derived from layered double hydroxides. Appl. Catal. A Gen. 2016, 517, 187–195. [Google Scholar] [CrossRef] [Green Version]















| Properties | Values |
|---|---|
| BET Surface Area | 147.7 m²/g |
| BJH Pore Volume | 0.615 cm³/g |
| BJH Pore Size | 13.7 nm |
| CO uptake in chemisorption | 56.2 umol/g |
| Dispersion | 14.0% |
| Metal surface area | 2.66 m2/g |
| Particle size | 9.37 nm |
| Pd wt % [EDS] | 4.3% |
| Ref | Catalysts | Methane Conversion, % | |
|---|---|---|---|
| (Metal loading, wt %) | 325 °C | 400 °C | |
| [a] | 5% Pd/γ-Al2O3 | 95 | 70 |
| [8] | 3% of Pd/Co3O4 on ZrO2 foams | 90 | 100 |
| [11] | 1% Pd on silicate monolith | 80 | 100 |
| [9] | 1% Pd@CeO2/γ-Al2O3 | 50 | 100 |
| [28] | 3% of Pd/Co3O4 on alumina foam | 35 | 95 |
| [29] | 5% Pd/Co3O4 | 30 | 90 |
| [3] | 3% Pd/γ-Al2O3 | 30 | 90 |
| [7] | 0.05% Pd-Co/Al2O3 | 20 | 90 |
| Run * | Methane Conversion, % |
|---|---|
| 1 | 94 |
| 2 | 94 |
| 3 | 92 |
| 4 | 90 |
| 5 | 92 |
| 6 | 90 |
| Reaction Conditions | Surface Pd, wt % | Surface Composition, wt % | |
|---|---|---|---|
| PdO | PdOx | ||
| Catalyst before reaction (Figure 7) | 1.64 | 38 | 62 |
| Catalyst after reaction at 325 °C (Figure 12) | 1.62 | 49 | 51 |
| Catalyst after reaction at 450 °C (Figure 14) | 1.62 | 68 | 32 |
| Pd nitrate calcined at 500 °C (Figure 13) | - | 100 | 0 |
| Pure PdO before and after reaction | - | 100 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, A.C.; McGuire, J.M.; Lawnick, O.; Bozack, M.J. Low-Temperature Activity and PdO-PdOx Transition in Methane Combustion by a PdO-PdOx/γ-Al2O3 Catalyst. Catalysts 2018, 8, 266. https://doi.org/10.3390/catal8070266
Banerjee AC, McGuire JM, Lawnick O, Bozack MJ. Low-Temperature Activity and PdO-PdOx Transition in Methane Combustion by a PdO-PdOx/γ-Al2O3 Catalyst. Catalysts. 2018; 8(7):266. https://doi.org/10.3390/catal8070266
Chicago/Turabian StyleBanerjee, Anil C., Jacqueline M. McGuire, Olivia Lawnick, and Michael. J. Bozack. 2018. "Low-Temperature Activity and PdO-PdOx Transition in Methane Combustion by a PdO-PdOx/γ-Al2O3 Catalyst" Catalysts 8, no. 7: 266. https://doi.org/10.3390/catal8070266
APA StyleBanerjee, A. C., McGuire, J. M., Lawnick, O., & Bozack, M. J. (2018). Low-Temperature Activity and PdO-PdOx Transition in Methane Combustion by a PdO-PdOx/γ-Al2O3 Catalyst. Catalysts, 8(7), 266. https://doi.org/10.3390/catal8070266