Laccase Activity as an Essential Factor in the Oligomerization of Rutin
Abstract
:1. Introduction
2. Results and Discussion
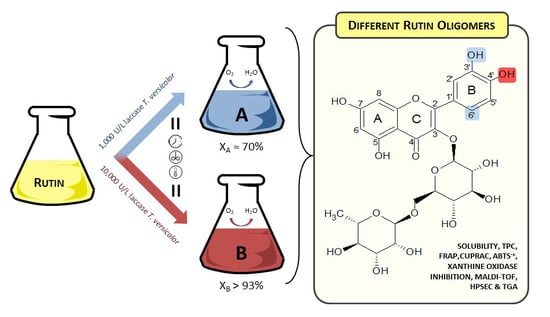
2.1. Polymerization Reaction
2.2. Apparent Solubility of Rutin Oligomers
2.3. Antioxidant Activities of Rutin Oligomer Fractions and Xanthine Oxidase Inhibitory Potential
2.4. Evaluation of the Phenolic Content of Rutin Polymerization Products
2.5. Molecular Weight Characterization of the Different Reaction Products
2.5.1. MALDI-TOF Analysis
2.5.2. HPSEC Analysis
2.6. Thermal Stability of Rutin Oligomers
3. Materials and Methods
3.1. Materials
3.2. Laccase Activity
3.3. Synthesis of Rutin Oligomers
3.3.1. Oligomerization Reaction
3.3.2. Separation and Lyophilization of the Polymers
3.4. Characterization
3.4.1. Apparent Solubility of Rutin Oligomers
3.4.2. Ferric Reducing Antioxidant Power (FRAP), Cupric Reducing Antioxidant Capacity (CUPRAC), ABTS+ Scavenging Activity and Xanthine Oxidase Inhibition Test
3.4.3. Assays for the Determination of Phenolic Compounds
3.4.4. Structural Analysis: MALDI-TOF and HPSEC
3.4.5. Production of Oligomers with Similar Molecular Mass
3.4.6. Thermal Stability Study of Rutin Oligomers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Romano, B.; Pagano, E.; Montanaro, V.; Fortunato, A.L.; Milic, N.; Borrelli, F. Novel insights into the pharmacology of flavonoids. Phyther. Res. 2013, 27, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Borges Bubols, G.; da Rocha Vianna, D.; Medina-Remón, A.; von Poser, G.; Lamuela-Raventos, R.M.; Eifler-Lima, V.L.; Cristina Garcia, S. The Antioxidant Activity of Coumarins and Flavonoids. Mini-Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Nassiri-Asl, M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J. Endocrinol. Investig. 2014, 37, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Rufa, L.; Yunning, Z.; Rui, W.; Jianying, L. A Study on the Extract of Tartary Buckwheat I. Toxicological Safety of the Extract of Tartary Buckwheat. Small 2001, 21, 602–607. [Google Scholar]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- NIH Dietary Supplement Label Database. Available online: https://www.dsld.nlm.nih.gov/dsld/index.jsp (accessed on 10 April 2018).
- Krewson, B.C.F.; Naghskit, J. Some Physical Properties of Rutin. J. Pharm. Sci. 1952, 41, 582–587. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Day, A.J.; Morgan, M.R.A. Experimental Determination of Octanol—Water Partition Coefficients of Quercetin and Related Flavonoids. J. Agric. Food Chem. 2005, 53, 4355–4360. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska, M.; Mizera, M.; Piotrowska, H.; Szymanowska-Powałowska, D.; Lewandowska, K.; Goscianska, J.; Pietrzak, R.; Bednarski, W.; Majka, Z.; Cielecka-Piontek, J. Complex of rutin with β-Cyclodextrin as potential delivery system. PLoS ONE 2015, 10, e0120858. [Google Scholar] [CrossRef] [PubMed]
- Chebil, L.; Rhouma, G.B.; Chekir-Ghedira, L.; Ghoul, M. Enzymatic Polymerization of Rutin and Esculin and Evaluation of the Antioxidant Capacity of Polyrutin and Polyesculin. In Biotechnology; Ekinci, D., Ed.; InTech: London, UK, 2015; pp. 117–133. ISBN 978-953-51-2040-7. [Google Scholar]
- Chung, J.E.; Kurisawa, M.; Kim, Y.J.; Uyama, H.; Kobayashi, S. Amplification of antioxidant activity of catechin by polycondensation with acetaldehyde. Biomacromolecules 2004, 5, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kurisawa, M.; Chung, J.E.; Uyama, H.; Kobayashi, S. Enzymatic synthesis and antioxidant properties of poly(rutin). Biomacromolecules 2003, 4, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Mouro, A.; Oliveira, I.M.; Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Comprehensive investigation of the enzymatic oligomerization of esculin by laccase in ethanol:water mixtures. RSC Adv. 2017, 7, 38424–38433. [Google Scholar] [CrossRef]
- Ghoul, M.; Chebil, L. Enzymatic polymerization of phenolic compounds by oxidoreductases. In SpringerBriefs in Molecular Science; Springer: Dordrecht, The Netherlands, 2012; ISBN 978-94-007-3918-5. [Google Scholar]
- Jeon, J.R.; Baldrian, P.; Murugesan, K.; Chang, Y.S. Laccase-catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, F.; Arends, I.W.C.E. Enzyme Initiated Radical Polymerizations. Polymers 2012, 4, 759–793. [Google Scholar] [CrossRef] [Green Version]
- Anthoni, J.; Lionneton, F.; Wieruszeski, J.M.; Magdalou, J.; Engasser, J.M.; Chebil, L.; Humeau, C.; Ghoul, M. Investigation of enzymatic oligomerization of rutin. Rasayan J. Chem. 2008, 1, 718–731. [Google Scholar]
- Rhouma, G.B.; Chebil, L.; Mustapha, N.; Krifa, M.; Ghedira, K.; Ghoul, M.; Chékir-Ghédira, L. Cytotoxic, genotoxic and antigenotoxic potencies of oligorutins. Hum. Exp. Toxicol. 2013, 32, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Bai, R.; Zhang, Y.; Wang, Q.; Fan, X.; Yuan, J.; Cui, L.; Wang, P. Laccase-Catalyzed Oxidative Polymerization of Phenolic Compounds. Appl. Biochem. Biotechnol. 2013, 171, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Burda, S.; Oleszek, W. Antioxidant and Antiradical Activities of Flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Vanden Berghe, D. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Darpan; Sharma, S.; Singh, R. Xanthine oxidase inhibitors: A patent survey. Expert Opin. Ther. Pat. 2011, 21, 1071–1108. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Kazuko, S.; Masaru, K.; Munehisa, A.; Mineo, S.; Naokata, M. Inhibition of Cow’s Milk Xanthine Oxidase by Flavonoids. J. Nat. Prod. 1988, 51, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Song, J.F.; Zhang, J.C. Determination of total phenols in environmental wastewater by flow-injection analysis with a biamperometric detector. Anal. Bioanal. Chem. 2002, 374, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Méchin, V.; Baumberger, S.; Pollet, B.; Lapierre, C. Peroxidase activity can dictate the in vitro lignin dehydrogenative polymer structure. Phytochemistry 2007, 68, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Process. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Anthoni, J.; Chebil, L.; Lionneton, F.; Magdalou, J.; Humeau, C.; Ghoul, M. Automated analysis of synthesized oligorutin and oligoesculin by laccase. Can. J. Chem. 2011, 89, 964–970. [Google Scholar] [CrossRef]
- Dávila, I.; Gullón, P.; Andrés, M.A.; Labidi, J. Coproduction of lignin and glucose from vine shoots by eco-friendly strategies: Toward the development of an integrated biorefinery. Bioresour. Technol. 2017, 244, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, Y.-S.; Shahgaldian, P.; Corvini, P.F.X.; Hommes, G. Sorption-assisted surface conjugation: A way to stabilize laccase enzyme. Appl. Microbiol. Biotechnol. 2011, 92, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Wariishi, H.; Valli, K.; Gold, M.H. Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium: Kinetic mechanism and role of chelators. J. Biol. Chem. 1992, 267, 23688–23695. [Google Scholar] [PubMed]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Optimization of solvent extraction of antioxidants from Eucalyptus globulus leaves by response surface methodology: Characterization and assessment of their bioactive properties. Ind. Crops Prod. 2017, 108, 649–659. [Google Scholar] [CrossRef]
- Boẑiĉ, M.; Gorgieva, S.; Kokol, V. Laccase-mediated functionalization of chitosan by caffeic and gallic acids for modulating antioxidant and antimicrobial properties. Carbohydr. Polym. 2012, 87, 2388–2398. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- EPA, U.S. Phenolics (Spectrophotometric, Manual 4-AAP with Distillation). Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-9065-phenolics-spectrophotometric-manual-4-aap-distillation (accessed on 4 August 2018).




| Fraction | Apparent Solubility | Fold |
|---|---|---|
| g/L | Relative to Controls | |
| AF3 | 6.76 ± 0.04 | ~58 |
| AF2 | 5.84 ± 0.40 | ~50 |
| AF1 | 2.21 ± 0.43 | ~19 |
| CA | 0.12 ± 0.01 | |
| BF3 | 10.77 ± 0.21 | ~71 |
| BF2 | 9.93 ± 0.28 | ~65 |
| BF1 | * | * |
| CB | 0.15 ± 0.01 |
| Fraction | FRAP | CUPRAC | ABTS+ | Xanthine Oxidase Inhibition |
|---|---|---|---|---|
| mg TE a/g Sample | mg TE a/g Sample | % Inhibition (1 g/L) | IC50 (mg/L) | |
| AF3 | 149.27 ± 22.67 | 488.30 ± 2.48 | 98 ± 1 | 186.36 ± 18.28 |
| AF2 | 138.02 ± 3.55 | 415.14 ± 7.43 | 95 ± 1 | 198.43 ± 14.90 |
| AF1 | 81.87 ± 1.93 | 245.67 ± 12.11 | 71 ± 2 | 372.30 ± 30.47 |
| CA | 168.79 ± 2.58 | 526.63 ± 8.81 | 100 ± 1 | 259.84 ± 1.45 |
| BF3 | 59.22 ± 1.29 | 226.21 ± 22.56 | 60 ± 3 | 241.28 ± 24.34 |
| BF2 | 13.33 ± 0.43 | 46.08 ± 3.44 | 11 ± 1 | > 400 b |
| BF1 | 12.11 ± 2.79 | 27.50 ± 1.10 | 8 ± 1 | > 600 b |
| CB | 109.37 ± 2.79 | 304.24 ± 65.76 | 85 ± 3 | 415.12 ± 2.72 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñiz-Mouro, A.; Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Laccase Activity as an Essential Factor in the Oligomerization of Rutin. Catalysts 2018, 8, 321. https://doi.org/10.3390/catal8080321
Muñiz-Mouro A, Gullón B, Lú-Chau TA, Moreira MT, Lema JM, Eibes G. Laccase Activity as an Essential Factor in the Oligomerization of Rutin. Catalysts. 2018; 8(8):321. https://doi.org/10.3390/catal8080321
Chicago/Turabian StyleMuñiz-Mouro, Abel, Beatriz Gullón, Thelmo A. Lú-Chau, María Teresa Moreira, Juan M. Lema, and Gemma Eibes. 2018. "Laccase Activity as an Essential Factor in the Oligomerization of Rutin" Catalysts 8, no. 8: 321. https://doi.org/10.3390/catal8080321
APA StyleMuñiz-Mouro, A., Gullón, B., Lú-Chau, T. A., Moreira, M. T., Lema, J. M., & Eibes, G. (2018). Laccase Activity as an Essential Factor in the Oligomerization of Rutin. Catalysts, 8(8), 321. https://doi.org/10.3390/catal8080321