Step-by-Step Growth of HKUST-1 on Functionalized TiO2 Surface: An Efficient Material for CO2 Capture and Solar Photoreduction
Abstract
:1. Introduction
2. Results and Discussion
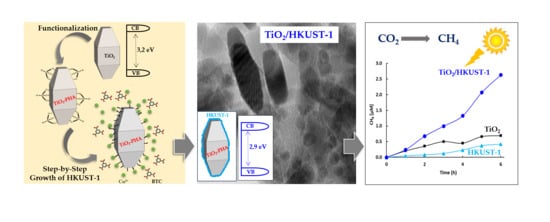
2.1. Spectroscopic and Morphological Characterization of TiO2-PHA and TiO2/HKUST-1
2.2. Photocatalytic Activity
2.3. Proposed Photocatalytic Pathway
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Shape-Controlled TiO2 NPs and Functionalization with PHA
3.3. Synthesis of TiO2/HKUST-1 Hybrid Catalyst
3.4. Characterization of TiO2 NPs, HKUST-1 and TiO2/HKUST-1
3.5. Photocatalytic CO2 Reduction
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chang, X.; Wang, T.; Gong, J. CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Monastersky, R. Global carbon dioxide levels near worrisome milestone. Nature 2013, 497, 13–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, A.C. Toward solar fuels: Photocatalytic conversion of carbon dioxide to hydrocarbons. ACS Nano 2010, 4, 1259–1278. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Huang, X.; Wang, X.; Wang, X. Progress in catalyst exploration for heterogeneous CO2 reduction and utilization: A critical review. J. Mater. Chem. A 2017, 5, 21625–21649. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- White, J.L.; Baruch, M.F.; Pander, J.E., III; Hu, Y.; Fortmeyer, I.C.; Park, J.E.; Zhang, T.; Liao, K.; Gu, J.; Yan, Y.; et al. Light-driven heterogeneous reduction of carbon dioxide: Photocatalysts and photoelectrodes. Chem. Rev. 2015, 115, 12888–12935. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ouyang, S.; Liu, L.; Reunchan, P.; Umezawa, N.; Ye, J. Recent advances in TiO2-based photocatalysis. J. Mater. Chem. A 2014, 2, 12642–12661. [Google Scholar] [CrossRef]
- Ringsmuth, A.K.; Landsberg, M.J.; Hankamer, B. Can photosynthesis enable a global transition from fossil fuels to solar fuels, to mitigate climate change and fuel-supply limitations? Renew. Sustain. Energy Rev. 2016, 62, 134–163. [Google Scholar] [CrossRef]
- Indrakanti, V.P.; Kubicki, J.D.; Schobert, H.H. Photoinduced activation of CO2 on Ti-based heterogeneous catalysts: Current state, chemical physics-based insights and outlook. Energy Environ. Sci. 2009, 2, 745–758. [Google Scholar] [CrossRef]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced photocatalytic CO2-reduction activity of anatase TiO2 by coexposed {001} and {101} facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Peng, T.; Ying, Z.; Song, S.; Zhang, J. Ag-loading on brookite TiO2 quasi nanocubes with exposed {210} and {001} facets: Activity and selectivity of CO2 photoreduction to CO/CH4. Appl. Catal. B Environ. 2016, 180, 130–138. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.; Jaroniec, M. Tunable photocatalytic selectivity of hollow TiO2 microspheres composed of anatase polyhedra with exposed {001} facets. J. Am. Chem. Soc. 2010, 132, 11914–11916. [Google Scholar] [CrossRef] [PubMed]
- Di Credico, B.; Bellobono, I.R.; D’Arienzo, M.; Fumagalli, D.; Redaelli, M.; Scotti, R.; Morazzoni, F. Efficacy of the reactive oxygen species generated by immobilized hydrothermal TiO2 in the photocatalytic degradation of diclofenac. Intern. J. Photoenergy 2015. [Google Scholar] [CrossRef]
- Anandan, S.; Yoon, M. Photocatalytic activities of the nano-sized TiO2-supported Y-zeolites. J. Photochem. Photobiol. C 2003, 4, 5–18. [Google Scholar] [CrossRef]
- Kang, C.; Jing, L.; Guo, T.; Cui, H.; Zhou, J.; Fu, H. Synthesis of high-temperature stable anatase TiO2 photocatalyst. J. Phys. Chem. C 2009, 213, 1006–1013. [Google Scholar] [CrossRef]
- Hu, P.; Morabito, J.V.; Tsung, C.K. Core−shell catalysts of metal nanoparticle core and metal−organic framework shell. ACS Catal. A 2014. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Zhu, C.; Liu, Y.; Cui, Y. Mesoporous metal–organic framework materials. Chem. Soc. Rev. 2012, 41, 1677–1695. [Google Scholar] [CrossRef] [PubMed]
- Rossin, A.; Di Credico, B.; Giambastiani, G.; Peruzzini, M.; Pescitelli, G.; Reginato, G.; Borfecchia, E.; Gianolio, G.; Lamberti, C.; Bordiga, S. Synthesis, characterization and CO2 uptake of a chiral Co(II) metal–organic framework containing a thiazolidine-based spacer. J. Mater. Chem. 2012, 22, 10335–10344. [Google Scholar] [CrossRef]
- Rossin, A.; Ienco, A.; Costantino, F.; Montini, T.; Di Credico, B.; Caporali, M.; Gonsalvi, L.; Fornasiero, P.; Peruzzini, M. Phase transitions and CO2 adsorption properties of polymeric magnesium formate. Cryst. Growth Des. 2008, 8, 3302–3308. [Google Scholar] [CrossRef]
- So, M.C.; Wiederrecht, G.P.; Mondloch, J.E.; Hupp, J.T.; Farha, O.K. Metal–organic framework materials for light-harvesting and energy transfer. Chem. Commun. 2015, 51, 3501–3505. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Ouyang, S.; Xu, H.; Zhao, M.; Zhang, X.; Ye, J. Co-ZIF-9/TiO2 nanostructure for superior CO2 photoreduction activity. Mater. Chem. A 2016, 4, 15126–15133. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Kafizas, A.; Zafeiratos, S.; Petit, C. CO2 capture and photocatalytic reduction using bifunctional TiO2/MOF nanocomposites under UV–vis irradiation. Appl. Catal. B Environ. 2017, 210, 131–140. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Multifunctional metal–organic frameworks for photocatalysis. Small 2015, 11, 3097–3112. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pi, Y.; Xia, Q.; Li, Z.; Xia, J. TiO2 encapsulated in Salicylaldehyde-NH2-MIL-101(Cr) for enhanced visible light-driven photodegradation of MB. Appl. Catal. B Environ. 2016, 191, 192–201. [Google Scholar] [CrossRef]
- Wang, D.; Huang, R.; Liu, W.; Sun, D.; Li, Z. Fe-based MOFs for photocatalytic CO2 reduction: Role of coordination unsaturated sites and dual excitation pathways. ACS Catal. 2014, 4, 4254–4260. [Google Scholar] [CrossRef]
- Bloch, E.D.; Britt, D.; Lee, C.; Doonan, C.J.; Uribe-Romo, F.J.; Furukawa, H.; Long, J.R.; Yaghi, O.M. Metal insertion in a microporous metal−organic framework lined with 2,2′-bipyridine. J. Am. Chem. Soc. 2010, 132, 14382–14384. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Gao, Y.; Fu, J.; Zeng, X.; Chen, Z.; Li, Z. Construction of a supported Ru complex on bifunctional MOF-253 for photocatalytic CO2 reduction under visible light. Chem. Commun. 2015, 51, 2645–2648. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Xu, C.; Qi, X.; Cao, Y.; Tang, J.; Zheng, Y.; Jiang, L. Highly efficient CuxO/TiO2 catalysts: Controllable dispersion and isolation of metal active species. Dalton Trans. 2016, 45, 4491–4495. [Google Scholar] [CrossRef] [PubMed]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. Chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Millward, A.R.; Yaghi, O.M. Metal−organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.M.; Shen, D.; Bulow, M.; Lau, M.L.; Deng, S.; Fitch, F.R.; OLemcoff, N.; Semanscin, J. Metallo-organic molecular sieve for gas separation and purification. Micropor. Mesopor. Mater. 2002, 55, 217–230. [Google Scholar] [CrossRef]
- Schlichte, K.; Kratzke, T.; Kaskel, S. Improved synthesis, thermal stability and catalytic properties of the metal-organic framework compound Cu3(BTC)2. Micropor. Mesopor. Mater. 2004, 3, 81–88. [Google Scholar] [CrossRef]
- Alaerts, L.; Seguin, E.; Poelman, H.; Thibault-Starzyk, F.; Jacobs, P.A.; De Vos, D.E. Probing the Lewis acidity and catalytic activity of the metal-organic framework [Cu3(BTC)2] (BTC = benzene-1,3,5-tricarboxylate). Chem. Eur. J. 2006, 12, 7353–7363. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hu, J.; Deng, M.; Wang, H.; Wang, X.; Hu, Y.; Jiang, H.L.; Jiang, J.; Zhang, Q.; Xie, Y.; et al. Integration of an Inorganic Semiconductor with a Metal–Organic Framework: A Platform for Enhanced Gaseous Photocatalytic Reactions. Adv. Mater. 2014, 26, 4783–4788. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Gan, Z.; Fisenko, S.; Wang, D.; El-Kaderi, H.M.; Wang, W.-N. Rapid formation of metal−organic frameworks (MOFs) based nanocomposites in microdroplets and their applications for CO2 photoreduction. ACS Appl. Mater. Interfaces 2017, 9, 9688–9698. [Google Scholar] [CrossRef] [PubMed]
- Anpo, M.; Thomas, J.M. Single site photocatalytic solids for the decomposition of undesirable molecules. Chem. Commun. 2006, 21, 3273–3278. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.; Yuan, Y.P.; Qiu, L.G.; Shen, Y.H.; Xie, A.J.; Zhu, J.F.; Tianc, X.Y.; Zhang, L.D. Facile fabrication of magnetic metal–organic framework nanocomposites for potential targeted drug delivery. J. Mater. Chem. 2011, 21, 3843–3848. [Google Scholar] [CrossRef]
- Anpo, M.; Yamashita, H.; Ichihashi, Y.; Ehara, S. Photocatalytic reduction of CO2 with H2O on various titanium oxide catalysts. J. Electroanal. Chem. 1995, 396, 21–26. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Dozzi, M.V.; Redaelli, M.; Di Credico, B.; Morazzoni, F.; Scotti, R.; Polizzi, S. crystal surfaces and fate of photogenerated defects in shape controlled anatase nanocrystals: Drawing useful relations to improve the H2 yield in methanol photosteam reforming. J. Phys. Chem. C 2015, 119, 12385–12393. [Google Scholar] [CrossRef]
- Ke, F.; Qiu, L.G.; Yuan, Y.P.; Jiang, X.; Zhu, J.F. Fe3O4@MOF core-shell magnetic microspheres with a designable metal–organic framework shell. J. Mater. Chem. 2012, 22, 9497–9500. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Carbajo, J.; Bahamonde, A.; Crippa, M.; Polizzi, S.; Scotti, R.; Wahba, L.; Morazzoni, F. Photogenerated defects in shape-controlled TiO2 anatase nanocrystals: A probe to evaluate the role of crystal facets in photocatalytic processes. J. Am. Chem. Soc. 2011, 133, 17652–17661. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, A.; Zhang, Q.; Yin, Y. Seed-mediated growth of anatase tio2 nanocrystals with core-antenna structures for enhanced photocatalytic activity. J. Am. Chem. Soc. 2015, 137, 11327–11339. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.; Schwerdtfeger, C.F.; Meagher, E.P. Refinement of the structure of anatase at several temperatures. Zeitschrift fuer Kristallographie 1972, 136, 273–281. [Google Scholar] [CrossRef]
- Summerfield, A.; Cebula, I.; Schröder, M.; Beton, P.H. Nucleation and early stages of layer-by-layer growth of metal organic frameworks on surfaces. J. Phys. Chem. C 2015, 119, 23544–23551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cubillas, P.; Anderson, M.W. Synthesis mechanism: Crystal growth and nucleation. In Zeolites and Catalysis: Synthesis, Reactions and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Guerrero, G.; Mutin, P.H.; Vioux, A. Anchoring of phosphonate and phosphinate coupling molecules on titania particles. Chem. Mater. 2001, 13, 4367–4373. [Google Scholar] [CrossRef]
- Prestipino, C.; Regli, L.; Vitillo, J.G.; Bonino, F.; Damin, A.; Lamberti, C.; Zecchina, A.; Solari, P.L.; Kongshaug, K.O.; Bordiga, S. Local structure of framework Cu(II) in HKUST-1 metallorganic framework: Spectroscopic characterization upon activation and interaction with adsorbates. Chem. Mater. 2006, 18, 1337–1346. [Google Scholar] [CrossRef]
- Dinh, C.T.; Nguyen, T.D.; Kleitz, F.; Do, T.O. Shape-controlled synthesis of highly crystalline titania nanocrystals. ACS Nano 2009, 3, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Cychosz, K.A. Physical adsorption characterization of nanoporous materials: Progress and challenges. Adsorption 2014, 20, 233–250. [Google Scholar] [CrossRef]
- Yan, X.; Komarneni, S.; Zhang, Z.; Yan, Z. Extremely enhanced CO2 uptake by HKUST-1 metal-organic frameworks via a simple chemical treatment. Micropor. Mesopor. Mater. 2014, 183, 69–73. [Google Scholar] [CrossRef]
- Wong-Foy, A.G.; Lebel, O.; Matzger, A.J. Porous crystal derived from a tricarboxylate linker with two distinct binding motifs. J. Am. Chem. Soc. 2007, 129, 15740–15741. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Suh, M.P. Selective CO2 adsorption in a flexible non-interpenetrated metal-organic framework. Chem. Commun. 2011, 47, 4258–4260. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xiang, S.; Arman, H.D.; Li, P.; Tidrow, S.; Zhao, D.; Chen, B. Microporous metal–organic framework with immobilized–oh functional groups within the pore surfaces for selective gas sorption. Eur. J. Inorg. Chem. 2010. [Google Scholar] [CrossRef]
- Shekhah, O.; Liu, J.; Fischer, R.A.; Woll, C. MOF thin films: Existing and future applications. Chem. Soc. Rev. 2011, 40, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Ma, L.L.; Li, J.L.; Yu, Y. In situ Fenton reagent generated from TiO2/Cu2O composite film: A new way to utilize TiO2 under visible light irradiation. Environ. Sci. Technol. 2007, 41, 6264–6269. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Li, Y.; Chen, X.; Tian, T.; Fang, P.; Zheng, F.; Zhao, X. Tuning the relative concentration ratio of bulk defects to surface defects in TiO2 nanocrystals leads to high photocatalytic efficiency. J. Am. Chem. Soc. 2011, 133, 16414–16417. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, H.; Grabstanowicz, L.; Gao, S.; Lou, Z.; Wang, W.; Huang, B.; Dai, Y.; Xu, T. Ti3+ self-doped TiO2−x anatase nanoparticles via oxidation of TiH2 in H2O2. Catal. Today 2014, 225, 80–89. [Google Scholar] [CrossRef]
- Abate, A.; Pérez-Tejada, R.; Wojciechowski, K.; Foster, J.M.; Sadhanala, A.; Steiner, U.; Snaith, H.J.; Franco, S.; Ordunac, J. Phosphonic anchoring groups in organic dyes for solid-state solar cells. Phys. Chem. Chem. Phys. 2015, 17, 18780–18789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellardita, M.; Di Paola, A.; García-López, E.; Loddo, V.; Marcì, G.; Palmisano, L. Photocatalytic CO2 reduction in gas-solid regime in the presence of bare, SiO2 supported or Cu-loaded TiO2 samples. Curr. Org. Chem. 2013, 17, 2440–2448. [Google Scholar] [CrossRef]
- Marcì, G.; García-López, E.I.; Palmisano, L. Photocatalytic CO2 reduction in gas–solid regime in the presence of H2O by using GaP/TiO2 composite as photocatalyst under simulated solar light. Catal. Commun. 2014, 53, 38–41. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Liu, L.; Zhang, Q.; Wang, J.; Li, Y. Photocatalytic conversion of CO2 and H2O to fuels by nanostructured Ce–TiO2/SBA-15 composites. Catal. Sci. Technol. 2012, 2, 2558–2568. [Google Scholar] [CrossRef]
- Liu, D.; Fernández, Y.; Ola, O.; Mackintosh, S.; Maroto-Valer, M.; Parlett, C.M.A.; Lee, A.F.; Wu, J.C.S. On the impact of Cu dispersion on CO2 photoreduction over Cu/TiO2. Catal. Commun. 2012, 25, 78–82. [Google Scholar] [CrossRef]
- Tseng, I.H.; Chang, W.C.; Wu, J.C.S. Photoreduction of CO2 using sol–gel derived titania and titania-supported copper catalysts. Appl. Catal. B Environ. 2002, 37, 37–48. [Google Scholar] [CrossRef]
- Sellaro, M.; Bellardita, M.; Brunetti, A.; Fontananova, E.; Palmisano, L.; Drioli, E.; Barbieri, G. CO2 conversion in a photocatalytic continuous membrane reactor. RSC Adv. 2016, 6, 67418–67427. [Google Scholar] [CrossRef]
- Mele, G.; Annese, C.; D’Accolti, L.; De Riccardis, A.; Fusco, C.; Palmisano, L.; Scarlino, A.; Vasapollo, G. Photoreduction of carbon dioxide to formic acid in aqueous suspension: A comparison between phthalocyanine/TiO2 and porphyrin/TiO2 catalysed processes. Molecules 2015, 20, 396–415. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, W.N.; Zhan, Z.; Woo, M.H.; Wu, C.Y.; Biswas, P. Photocatalytic reduction of CO2 with H2O on mesoporous silica supported Cu/TiO2 catalyst. Appl. Catal. B Environ. 2010, 100, 386–392. [Google Scholar] [CrossRef]
- Mei, B.; Pougin, A.; Strunk, J. Influence of photodeposited gold nanoparticles on the photocatalytic activity of titanate species in the reduction of CO2 to hydrocarbons. J. Catal. 2013, 306, 184–189. [Google Scholar] [CrossRef]
- Mousavi, M.; Habibi-Yangjeh, A.; Abitorabi, M. Fabrication of novel magnetically separable nanocomposites using graphitic carbon nitride, silver phosphate and silver chloride and their applications in photocatalytic removal of different pollutants using visible-light irradiation. J. Colloid Interface Sci. 2016, 480, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Morrison, S.R. Electrochemistry at Semiconductor and Oxidized Metal Electrode; Plenum Press: New York, NY, USA, 1980. [Google Scholar]
- Sanderson, R.T. Chemical Periodicity; Reinhold Pub. Corp.: New York, NY, USA, 1960. [Google Scholar]
- Serpone, N.; Pelizzetti, E. Photocatalysis, Fundamentals and Applications; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Ikeda, S.; Sugiyama, N.; Muratami, S.; Kominami, H.; Kera, Y.; Noguchi, H.; Uosaki, K.; Torimoto, T.; Ohtani, B. Quantitative analysis of defective sites in titanium(IV) oxide photocatalyst powders. Phys. Chem. Chem. Phys. 2003, 5, 778–783. [Google Scholar] [CrossRef] [Green Version]
- Leytner, S.; Hupp, J.T. Evaluation of the energetics of electron trap states at the nanocrystalline titanium dioxide/aqueous solution interface via time-resolved photoacoustic spectroscopy. Chem. Phys. Lett. 2000, 330, 231–236. [Google Scholar] [CrossRef]
- Abdellah, M.; El-Zohry, A.M.; Antila, L.J.; Windle, C.D.; Reisner, E.; Hammarström, L. Time-resolved IR spectroscopy reveals a mechanism with TiO2 as a reversible electron acceptor in a TiO2−re catalyst system for CO2 photoreduction. J. Am. Chem. Soc. 2017, 139, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.S.; Zou, L.; Hu, E. Photocatalytic reduction of carbon dioxide into gaseous hydrocarbon using TiO2 pellets. Catal. Today 2006, 115, 269–273. [Google Scholar] [CrossRef]
- Zhang, Q.; Rao, G.; Rogers, J.; Zhao, C.; Liu, L.; Li, Y. Novel anti-fouling Fe2O3/TiO2 nanowire membranes for humic acid removal from water. Chem. Eng. J. 2015, 271, 180–187. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Slamet, H.W.N.; Ezza, P.; Kapti, R.; Jarnuzi, G. Effect of copper species in a photocatalytic synthesis of methanol from carbon dioxide over copper-doped titania catalysts. World Appl. Sci. J. 2009, 6, 112–122. [Google Scholar]
- Altomare, A.; Corriero, N.; Cuocci, C.; Falcicchio, A.; Moliterni, A.; Rizzi, R. QUALX2.0: A qualitative phase analysis software using the freely available database POW_COD. J. Appl. Cryst. 2015, 48, 598–603. [Google Scholar] [CrossRef]
- Briggs, D.; Seah, M.P. Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; John Wiley and Sons Ltd.: Chichester, UK, 1983. [Google Scholar]
- Shirley, D.A. High-resolution X-Ray photoemission spectrum of the valence bands of gold. Phys. Rev. B Condens. Matter 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lauac, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Kaushik, V.K. Identification of oxidation states of copper in mixed oxides and chlorides using ESCA1. Spectrochim. Acta Part B 1989, 44, 581–587. [Google Scholar] [CrossRef]











© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Credico, B.; Redaelli, M.; Bellardita, M.; Calamante, M.; Cepek, C.; Cobani, E.; D’Arienzo, M.; Evangelisti, C.; Marelli, M.; Moret, M.; et al. Step-by-Step Growth of HKUST-1 on Functionalized TiO2 Surface: An Efficient Material for CO2 Capture and Solar Photoreduction. Catalysts 2018, 8, 353. https://doi.org/10.3390/catal8090353
Di Credico B, Redaelli M, Bellardita M, Calamante M, Cepek C, Cobani E, D’Arienzo M, Evangelisti C, Marelli M, Moret M, et al. Step-by-Step Growth of HKUST-1 on Functionalized TiO2 Surface: An Efficient Material for CO2 Capture and Solar Photoreduction. Catalysts. 2018; 8(9):353. https://doi.org/10.3390/catal8090353
Chicago/Turabian StyleDi Credico, Barbara, Matteo Redaelli, Marianna Bellardita, Massimo Calamante, Cinzia Cepek, Elkid Cobani, Massimiliano D’Arienzo, Claudio Evangelisti, Marcello Marelli, Massimo Moret, and et al. 2018. "Step-by-Step Growth of HKUST-1 on Functionalized TiO2 Surface: An Efficient Material for CO2 Capture and Solar Photoreduction" Catalysts 8, no. 9: 353. https://doi.org/10.3390/catal8090353
APA StyleDi Credico, B., Redaelli, M., Bellardita, M., Calamante, M., Cepek, C., Cobani, E., D’Arienzo, M., Evangelisti, C., Marelli, M., Moret, M., Palmisano, L., & Scotti, R. (2018). Step-by-Step Growth of HKUST-1 on Functionalized TiO2 Surface: An Efficient Material for CO2 Capture and Solar Photoreduction. Catalysts, 8(9), 353. https://doi.org/10.3390/catal8090353