Recent Advances in the Catalytic Synthesis of Imidazolidin-2-ones and Benzimidazolidin-2-ones
Abstract
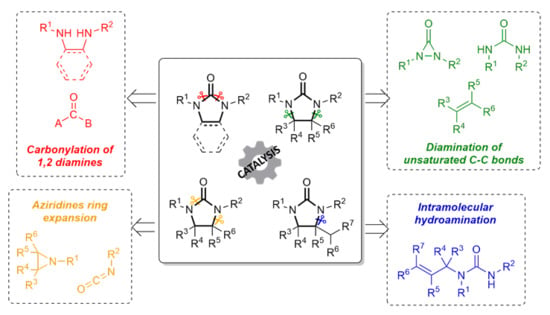
:1. Introduction
2. Synthesis of 2-(benz)imidazolidinones by Direct Incorporation of the Carbonyl Group into 1,2-Diamines
3. Synthesis of 2-Imidazolidinones by Catalytic Diamination of Unsaturated C–C Bonds
3.1. Metal-Catalyzed Intramolecular Diamination of Alkenes with Ureas
3.2. Metal-Catalyzed Intramolecular Diamination of Allenes with Ureas
3.3. Metal-Catalyzed Intramolecular Diamination of Alkynes with Ureas
3.4. Metal-Catalyzed Intermolecular Diamination of Dienes with Ureas
3.5. Metal-Catalyzed Intermolecular Diamination of Alkenes with Diaziridinones
4. Synthesis of 2-Imidazolidinone Derivatives by Catalytic Hydroamination of Unsaturated Ureas
4.1. Intramolecular N-hydroamination of Propargylic Ureas
4.2. Intramolecular N-hydroamination of Allylic Ureas
5. Catalytic Aziridine Ring Expansion
5.1. Palladium-Catalyzed Aziridine Ring Expansion Reactions
5.2. Nickel-Catalyzed Aziridine Ring Expansion Reactions
5.3. Other Catalytic Aziridine Ring-Expansion Reactions
5.4. Non-Catalytic Aziridine Ring-Expansion Reactions
6. Miscellaneous
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| BINAP | 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl |
| BINOL | [1,1′-binaphthalene]-2,2′-diol |
| BQ | 1,4-Benzoquinone |
| DCE | 1,2-Dichloroethane |
| DCM | Dichloromethane |
| DIPEA | N,N-Diisopropylethylamine |
| DMF | N,N-Dimethylformamide |
| MeCN or ACN | Acetonitrile |
| MS | Molecular sieves |
| NHC | N-heterocyclic carbenes |
| NMP | N-Methyl-2-pyrrolidone |
| PhI(OAc)2 | (Diacetoxyiodo)benzene |
| RNA | Ribonucleic acid |
| rt or RT | Room temperature |
| TBAF | Tetra-n-butylammonium fluoride |
| TEMPO | 2,2,6,6-Tetramethyl-1-piperidinyloxy, free radical |
| Tf | Triflate |
| THF | Tetrahydrofuran |
| TBD | 1,5,7-Triazabicyclo[4.4.0]dec-5-ene |
| MTBD | 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene |
| VOC | Volatile Organic Compound |
References
- Lee, K.-C.; Venkateswararao, E.; Sharma, V.K.; Jung, S.-H. Investigation of amino acid conjugates of (S)-1-[1-(4-aminobenzoyl)-2,3-dihydro-1H-indol-6-sulfonyl]-4-phenyl-imidazolidin-2-one (DW2282) as water soluble anticancer prodrugs. Eur. J. Med. Chem. 2014, 80, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; McNulty, J.; Herald, D.L.; Doubek, D.L.; Chapuis, J.-C.; Schmidt, J.M.; Tackett, L.P.; Boyd, M.R. Antineoplastic Agents. 362. Isolation and X-ray Crystal Structure of Dibromophakellstatin from the Indian Ocean Sponge Phakellia mauritiana. J. Nat. Prod. 1997, 60, 180–183. [Google Scholar] [CrossRef]
- Chang, C.-S.; Lin, Y.-T.; Shih, S.-R.; Lee, C.-C.; Lee, Y.-C.; Tai, C.-L.; Tseng, S.-N.; Chern, J.-H. Design, Synthesis, and Antipicornavirus Activity of 1-[5-(4-Arylphenoxy)alkyl]-3-pyridin-4-ylimidazolidin-2-one Derivatives. J. Med. Chem. 2005, 48, 3522–3535. [Google Scholar] [CrossRef] [PubMed]
- Reichard, G.A.; Stengone, C.; Paliwal, S.; Mergelsberg, I.; Majmundar, S.; Wang, C.; Tiberi, R.; McPhail, A.T.; Piwinski, J.J.; Shih, N.-Y. Asymmetric Synthesis of 4,4-Disubstituted-2-Imidazoli-dinones: Potent NK 1 Antagonists. Org. Lett. 2003, 5, 4249–4251. [Google Scholar] [CrossRef]
- Kaneko, H.; Ikawa, T.; Yamamoto, Y.; Arulmozhiraja, S.; Tokiwa, H.; Akai, S. 3-(Triflyloxy)benzynes Enable the Regiocontrolled Cycloaddition of Cyclic Ureas to Synthesize 1,4-Benzodiazepine Derivatives. Synlett 2018, 29, 943–948. [Google Scholar]
- Rajesh, M.; Puri, S.; Kant, R.; Sridhar Reddy, M. Ag-Catalyzed Intramolecular Sequential Vicinal Diamination of Alkynes with Isocyanates: Synthesis of Fused Indole-Cyclic Urea Derivatives. J. Org. Chem. 2017, 82, 5169–5177. [Google Scholar] [CrossRef] [PubMed]
- Koswatta, P.; Sivappa, R.; Dias, H.; Lovely, C. Total Synthesis of the Leucetta-Derived Alkaloid Calcaridine A. Synthesis 2009, 2009, 2970–2982. [Google Scholar]
- Vasanthakumar, G.R.; Bhor, V.M.; Surolia, A. Hydrolysis of Cyclic Ureas under Microwave Irradiation: Synthesis and Characterization of 7,8-Diaminopelargonic Acid. Synth. Commun. 2007, 37, 2633–2639. [Google Scholar] [CrossRef]
- Barrios Sosa, A.C.; Yakushijin, K.; Horne, D.A. Synthesis of Slagenins A, B, and C. Org. Lett. 2000, 2, 3443–3444. [Google Scholar] [CrossRef] [PubMed]
- Hodges, T.R.; Abbott, J.R.; Little, A.J.; Sarkar, D.; Salovich, J.M.; Howes, J.E.; Akan, D.T.; Sai, J.; Arnold, A.L.; Browning, C.; et al. Discovery and Structure-Based Optimization of Benzimidazole-Derived Activators of SOS1-Mediated Nucleotide Exchange on RAS. J. Med. Chem. 2018, 61, 8875–8894. [Google Scholar] [CrossRef]
- Lu, C.; Hu, L.; Yang, G.; Chen, Z. 2-Imidazolidinones as Chiral Auxiliaries in Asymmetric Synthesis. Curr. Org. Chem. 2012, 16, 2802–2817. [Google Scholar] [CrossRef]
- Guillena, G.; Nájera, C. (4R,5S)-1,5-Dimethyl-4-phenylimidazolidin-2-one as a chiral auxiliary for the diastereoselective alkylation of a new iminic glycine derivative: practical asymmetric synthesis of α-amino acids. Tetrahedron: Asymmetry 1998, 9, 1125–1129. [Google Scholar] [CrossRef]
- Matsunaga, H.; Ishizuka, T.; Kunieda, T. Synthetic utility of five-membered heterocycles—chiral functionalization and applications. Tetrahedron 2005, 61, 8073–8094. [Google Scholar] [CrossRef]
- Konnert, L.; Lamaty, F.; Martinez, J.; Colacino, E. Recent Advances in the Synthesis of Hydantoins: The State of the Art of a Valuable Scaffold. Chem. Rev. 2017, 117, 13757–13809. [Google Scholar] [CrossRef] [PubMed]
- Antonova, M.M.; Baranov, V.V.; Kravchenko, A.N. Methods for the synthesis of 1-substituted 1H-imidazol-2(3H)-ones. Chem. Heterocycl. Compd. 2015, 51, 395–420. [Google Scholar] [CrossRef]
- Kou, Q.; Wang, T.; Zou, F.; Zhang, S.; Chen, Q.; Yang, Y. Design, synthesis and biological evaluation of C(4) substituted monobactams as antibacterial agents against multidrug-resistant Gram-negative bacteria. Eur. J. Med. Chem. 2018, 151, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, Z.; Knipe, P.C. Conformationally Programmable Chiral Foldamers with Compact and Extended Domains Controlled by Monomer Structure. Angew. Chem. Int. Ed. 2018, 57, 8478–8482. [Google Scholar] [CrossRef]
- Remarchuk, T.; Corey, E.J. Stereodivergent synthesis of novel chiral C2-symmetric bis-trifluoromethyl-2-oxazolidinones. Tetrahedron Lett. 2018, 59, 2256–2259. [Google Scholar] [CrossRef]
- Ramadoss, V.; Alonso-Castro, A.J.; Campos-Xolalpa, N.; Solorio-Alvarado, C.R. Protecting-Group-Free Total Synthesis and Biological Evaluation of 3-Methylkealiiquinone and Structural Analogues. J. Org. Chem. 2018, 83, 10627–10635. [Google Scholar] [CrossRef]
- Ramadoss, V.; Alonso-Castro, A.J.; Campos-Xolalpa, N.; Ortiz-Alvarado, R.; Yahuaca-Juárez, B.; Solorio-Alvarado, C. Total synthesis of kealiiquinone: the regiocontrolled strategy for accessing its 1-methyl-4-arylbenzimidazolone core. RSC Adv. 2018, 8, 30761–30776. [Google Scholar] [CrossRef]
- Monaim, S.A.H.A.; Acosta, G.A.; Royo, M.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Solid-phase synthesis of homodetic cyclic peptides from Fmoc-MeDbz-resin. Tetrahedron Lett. 2018, 59, 1779–1782. [Google Scholar] [CrossRef]
- Spengler, J.; Blanco-Canosa, J.B.; Forni, L.; Albericio, F. One-Pot Peptide Ligation−Oxidative Cyclization Protocol for the Preparation of Short-/Medium-Size Disulfide Cyclopeptides. Org. Lett. 2018, 20, 4306–4309. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Kurotani, R.; Konno, H. Synthesis of a secretoglobin 3A2 type C (98–139) fragment by Dawson’s native chemical ligation. Tetrahedron Lett. 2017, 58, 4145–4148. [Google Scholar] [CrossRef]
- Teno, N.; Iguchi, Y.; Yamashita, Y.; Mori, N.; Une, M.; Nishimaki-Mogani, T.; Gohda, K. Discovery and optimization of benzimidazole derivatives as a novel chemotype of farnesoid X receptor (FXR) antagonists. Biorg. Med. Chem. 2017, 25, 1787–1794. [Google Scholar] [CrossRef]
- Jbara, M.; Maity, S.K.; Seenaiah, M.; Brik, A. Palladium Mediated Rapid Deprotection of N-Terminal Cysteine under Native Chemical Ligation Conditions for the Efficient Preparation of Synthetically Challenging Proteins. J. Am. Chem. Soc. 2016, 138, 5069–5075. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Sun, Z.; Xu, Y.-J.; Lu, C.-D. Synthesis of Aryl anti-Vicinal Diamines via Aza-Brook Rearrangement-Initiated Nucleophilic Addition of α-Silylamines to Imines. J. Org. Chem. 2015, 80, 3714–3722. [Google Scholar] [CrossRef] [PubMed]
- Stanchev, S.; Zawada, Z.; Monincová, L.; Bednárová, L.; Slaninová, J.; Fučík, V.; Čeřovský, V. Synthesis of lucifensin by native chemical ligation and characteristics of its isomer having different disulfide bridge pattern. J. Pept. Sci. 2014, 20, 725–735. [Google Scholar] [CrossRef]
- Chavan, S.P.; Chavan, P.N.; Lasonkar, P.B.; Khairnar, L.B.; Kadam, A.L. A Facile and Convenient Synthesis of (±)-Biotin via MgCl2/Et3N-Mediated C–C Coupling and Mitsunobu Reaction. Synlett 2014, 25, 2879–2882. [Google Scholar] [CrossRef]
- Pajkert, R.; Böttcher, T.; Ponomarenko, M.; Bremer, M.; Röschenthaler, G.-V. Synthesis and characterization of novel carbene complexes of phosphorus(V) fluorides with potential liquid-crystalline properties. Tetrahedron 2013, 69, 8943–8951. [Google Scholar] [CrossRef]
- Lee, J.-H.; Thanigaimalai, P.; Lee, K.-C.; Bang, S.-C.; Kim, M.-S.; Sharma, V.K.; Yun, C.-Y.; Roh, E.; Kim, Y.; Jung, S.-H. Novel Benzo[d]imidazole-2(3H)-thiones as Potent Inhibitors of the α-Melanocyte Stimulating Hormone Induced Melanogenesis in Melanoma B16 Cells. Chem. Pharm. Bull. 2010, 918–921. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhu, J. Organocatalytic Nucleophilic Addition of Hydrazones to Imines: Synthesis of Enantioenriched Vicinal Diamines. Angew. Chem. Int. Ed. 2017, 56, 5612–5615. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Wilcox, C.B.; Unruh, D.K.; Jiang, B.; Li, G. Asymmetric [3 + 2] Cycloaddition of Chiral N-Phosphonyl Imines with Methyl Isocyanoacetate for Accessing 2-Imidazolines with Switchable Stereoselectivity. J. Org. Chem. 2017, 82, 2992–2999. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Yan, M.; Baran, P.S. 11-Step Total Synthesis of Araiosamines. J. Am. Chem. Soc. 2016, 138, 14234–14237. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.E.; Kim, B.R.; Sung, G.H.; Yoon, H.J.; Yoon, Y.-J. Facile Synthesis of Benzo[d]azol-2(3H)-ones Using 2-Phenoxycarbonyl-4,5-dichloropyridazin-3(2H)-one as Green CO Source. Synlett 2015, 26, 1985–1990. [Google Scholar] [CrossRef]
- Tolpygin, I.E.; Popova, O.S.; Revinskii, Y.V.; Dubonosov, A.D.; Bren’, V.A.; Minkin, V.I. Carbamides as Chemosensors for Cations Eu3+. Russ. J. Org. Chem. 2013, 49, 1238–1240. [Google Scholar] [CrossRef]
- Dekhane, D.V.; Pawar, S.S.; Gupta, S.V.; Shingare, M.S.; Thore, S.N. Synthesis of Benzimidazolones, Benzooxazolones, 2-amino-benzothiazoles from Ethyl Cyanoformate and o-phenylene Diamines, o-aminophenols, oaminothiophenols Promoted by Lithium Bromide. Lett. Org. Chem. 2011, 8, 406–411. [Google Scholar] [CrossRef]
- Adardour, M.; Zaballos-García, E.; Loughzail, M.; Dahaoui, S.; Baouid, A. Synthesis, characterization and X-ray structure of heterocyclic systems prepared via 1,3-dipolar cycloaddition of nitrile oxides with benzimidazolone. J. Mol. Struct. 2018, 1165, 153–161. [Google Scholar] [CrossRef]
- Wei, S.; Li, L.; Shu, Y.; Zhao, Y.; Ji, Z. Synthesis, antifungal and antitumor activity of two new types of imidazolin-2-ones. Bioorg. Med. Chem. 2017, 25, 6501–6510. [Google Scholar] [CrossRef]
- Yang, F.; Wu, C.; Li, Z.; Tian, G.; Wu, J.; Zhu, F.; Zhang, J.; He, Y.; Shen, J. A Facile Route of Synthesis for Making Flibanserin. Org. Process Res. Dev. 2016, 20, 1576–1580. [Google Scholar] [CrossRef]
- Mondieig, D.; Negrier, P.; Leger, J.M.; Lakhrissi, L.; El Assyry, A.; Lakhrissi, B.; Essassi, E.M.; Benali, B.; Boucetta, A. Synthesis and Structural Study of N-Isopropenylbenzimidazolone. Russ. J. Phys. Chem. A 2015, 89, 807–811. [Google Scholar] [CrossRef]
- Rekunge, D.S.; Khatri, C.K.; Chaturbhuj, G.U. Sulfated polyborate-catalyzed efficient and expeditious synthesis of (un) symmetrical ureas and benzimidazolones. Tetrahedron Lett. 2017, 58, 4304–4307. [Google Scholar] [CrossRef]
- Movsumzade, M.M.; Shatirova, M.I.; Dzhafarova, U.S.; Niyazova, N.K. Investigation of Chemical Properties and Antimicrobial Activity of Acetylene Glycidyl Ethers. Russ. J. Org. Chem. 2018, 88, 389–392. [Google Scholar] [CrossRef]
- Devine, W.G.; Diaz-Gonzalez, R.; Ceballos-Perez, G.; Rojas, D.; Satoh, T.; Tear, W.; Ranade, R.M.; Barros-Álvarez, X.; Hol, W.G.J.; Buckner, F.S.; et al. From Cells to Mice to Target: Characterization of NEU-1053 (SB-443342) and Its Analogues for Treatment of Human African Trypanosomiasis. ACS Infect. Dis. 2017, 3, 225–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Mu, A.U.; Lin, Y.-H.; Guo, Z.-H.; Yuan, T.; Wheeler, S.E.; Fang, L. Molecular Coplanarity and Self-Assembly Promoted by Intramolecular Hydrogen Bonds. Org. Lett. 2016, 18, 6332–6335. [Google Scholar] [CrossRef] [PubMed]
- Asteian, A.; Blayo, A.-L.; He, Y.; Koenig, M.; Shin, Y.; Kuruvilla, D.S.; Corzo, C.A.; Cameron, M.D.; Lin, L.; Ruiz, C.; et al. Design, Synthesis and Biological Evaluation of Indole Biphenylcarboxylic acids as PPARγ Antagonists. ACS Med. Chem. Lett. 2015, 6, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.A.; Hameed, S.; Farman, M.; Kressler, J.; Mahmood, N. Conjugates of degraded and oxidized hydroxyethyl starch and sulfonylureas: synthesis, characterization, and in vivo antidiabetic activity. Bioconjug. Chem. 2015, 26, 120–127. [Google Scholar]
- Wu, J.-W.; Wu, Y.-D.; Dai, J.-J.; Xu, H.-J. Benzoic Acid-Catalyzed Transamidation Reactions of Carboxamides, Phthalimide, Ureas and Thioamide with Amines. Adv. Synth. Catal. 2014, 356, 2429–2436. [Google Scholar] [CrossRef]
- Abbas, M.A.; Hameed, S.; Kressler, J. Preparation of 2(3H)-Benzimidazolone and its Derivative Under Aqueous Condition As a Potential Agent for Antidiabetic Compounds. Asian J. Org. Chem. 2013, 25, 509–511. [Google Scholar] [CrossRef]
- Francavilla, C.; Turtle, E.D.; Kim, B.; O’Mahony, D.J.R.; Shiau, T.P.; Low, E.; Alvarez, N.J.; Celeri, C.E.; D’Lima, L.; Friedman, L.C.; et al. Novel N-chloroheterocyclic antimicrobials. Bioorg. Med. Chem. Lett. 2011, 21, 3029–3033. [Google Scholar] [CrossRef]
- Humphries, P.S.; Bersot, R.; Kincaid, J.; Mabery, E.; McCluskie, K.; Park, T.; Renner, T.; Riegler, E.; Steinfeld, T.; Turtle, E.D.; et al. Carbazole-containing amides and ureas: Discovery of cryptochrome modulators as antihyperglycemic agents. Bioorg. Med. Chem. Lett. 2018, 28, 293–297. [Google Scholar] [CrossRef]
- Periasamy, J.; Kurdekar, V.; Jasti, S.; Nijaguna, M.; Boggaram, S.; Hurakadli, M.A.; Raina, D.; Kurup, L.M.; Chintha, C.; Manjunath, K.; et al. Targeting Phosphopeptide Recognition by the Human BRCA1 Tandem BRCT Domain to Interrupt BRCA1-Dependent Signaling. Cell Chem. Biol. 2018, 25, 677–690. [Google Scholar]
- Chen, Z.; Mori, W.; Zhang, X.; Yamasaki, T.; Dunn, P.J.; Zhang, G.; Fu, H.; Shao, T.; Zhang, Y.; Hatori, A.; et al. Synthesis, pharmacology and preclinical evaluation of 11C-labeled 1,3-dihydro-2H-benzo[d]imidazole-2-ones for imaging γ8-dependent transmembrane AMPA receptor regulatory protein. Eur. J. Med. Chem. 2018, 157, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; He, H.; Ye, Z.; Zhu, K.; Wu, Y.; Zhang, F. Highly Selective Palladium-Catalyzed Arene C−H Acyloxylation with Benzothiadiazole as a Modifiable Directing Group. Org. Lett. 2018, 20, 5692–5695. [Google Scholar]
- Vincent, J.; Adura, C.; Gao, P.; Luz, A.; Lama, L.; Asano, Y.; Okamoto, R.; Imaeda, T.; Aida, J.; Rothamel, K.; et al. Small molecule inhibition of cGAS reduces interferon expression in primary macrophages from autoimmune mice. Nature Commun. 2018, 8, 750. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kim, J.H.; Koh, Y.; Tran, P.-T.; Ann, J.; Yoon, I.; Jang, J.; Kim, W.K.; Lee, S.; Lee, J.; et al. Discovery of simplified leucyladenylate sulfamates as novel leucyl-tRNA synthetase (LRS)-targeted mammalian target of rapamycin complex 1 (mTORC1) inhibitors. Bioorg. Med. Chem. 2017, 25, 4145–4152. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-H.; Lu, Y.; Zhang, Q.; Lu, R.; Bao, L.-Q.; Shen, M.-H.; Xu, H.-D. Selective S-arylation of 2-oxazolidinethiones and selective N-arylation of 2-benzoxazolinones/2-benzimidazolinones. Org. Biomol. Chem. 2017, 15, 4058–4063. [Google Scholar] [CrossRef] [PubMed]
- Garlets, Z.J.; Parenti, K.R.; Wolfe, J.P. Asymmetric Palladium-Catalyzed Alkene Carboamination Reactions for the Synthesis of Cyclic Sulfamides. Chem. Eur. J. 2016, 22, 5919–5922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, C.E.; Ramsey, J.D.; McAtee, C.C.; Mauck, J.R.; Hale, E.M.; Cumens, J.A. The Use of Ureates as Activators for Samarium Diiodide. J. Org. Chem. 2016, 81, 5903–5914. [Google Scholar] [CrossRef] [PubMed]
- Alberti, M.N.; Polyhach, Y.; Tzirakis, M.D.; Tçdtli, L.; Jeschke, G.; Diederich, F. Exploring the Strength of the H-Bond in Synthetic Models for Heme Proteins: The Importance of the N¢H Acidity of the Distal Base. Chem. Eur. J. 2016, 22, 10194–10202. [Google Scholar] [CrossRef]
- Hesp, C.R.; MacDonald, M.J.; Zahedi, M.M.; Bilodeau, D.A.; Zhao, S.-B.; Pesant, M.; Beauchemin, A.M. Formaldehyde as Tethering Organocatalyst: Highly Diastereoselective Hydroaminations of Allylic Amines. Org. Lett. 2015, 17, 5136–5139. [Google Scholar] [CrossRef]
- Doveston, R.G.; Tosatti, P.; Dow, M.; Foley, D.J.; Li, H.Y.; Campbell, A.J.; House, D.; Churcher, I.; Marsden, S.P.; Nelson, A. A unified lead-oriented synthesis of over fifty molecular molecular scaffolds. Org. Biomol. Chem. 2015, 13, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Neudorfer, C.; Seddik, A.; Shanab, K.; Jurik, A.; Rami-Mark, C.; Holzer, W.; Ecker, G.; Mitterhauser, M.; Wadsak, W.; Spreitzer, H. Synthesis and in Silico Evaluation of Novel Compounds for PET-Based Investigations of the Norepinephrine Transporter. Molecules 2015, 20, 1712–1720. [Google Scholar] [CrossRef]
- Badru, R.; Singh, B. Synthesis of imidazolidin-2-ones employing dialkyl carbonates as an ecofriendly carbonylation source. RSC Adv. 2014, 4, 38978–38985. [Google Scholar] [CrossRef]
- Liu, J.; Xie, Y.; Ye, L.; Yan, H.; Tu, S. Efficient and Green Preparation of 2-Imidazolidinone using Sulfamic Acid as Acidic Catalyst. Org. Prep. Proced. Int. 2014, 46, 453–456. [Google Scholar] [CrossRef]
- Gabriele, B.; Costa, M.; Salerno, G.; Chiusoli, G.P. A New Synthesis of Trimethyl Aconitate by Palladium-catalysed Triple Carbonylation of Propynyl Alcohol. J. Chem. Soc., Chem. Commun. 1992, 1007–1008. [Google Scholar] [CrossRef]
- Gabriele, B.; Costa, M.; Salerno, G.; Chiusoli, G.P. An Efficient and Selective Palladium-catalysed Oxidative Dicarbonylation of Alkynes to Alkyl- or Aryl-maleic Esters. J. Chem. Soc., Perkin Trans. 1 1994, 83–87. [Google Scholar] [CrossRef]
- Gabriele, B. Synthesis of Heterocycles by Palladium-Catalyzed Carbonylative Reactions. In Advances in Transition-Metal Mediated Heterocyclic Synthesis; Solé, D., Fernández, I., Eds.; Academic Press-Elsevier: London, UK, 2018; pp. 55–127. [Google Scholar]
- Gabriele, B.; Salerno, G.; Costa, M. Oxidative Carbonylations. Top. Organomet. Chem. 2006, 18, 239–272. [Google Scholar]
- Gabriele, B.; Salerno, G. PdI2. In e-EROS (Electronic Encyclopedia of Reagents for Organic Synthesis); Crich, D., Ed.; Wiley-Interscience: New York, NY, USA, 2006. [Google Scholar]
- Mancuso, R.; Ziccarelli, I.; Chimento, A.; Marino, N.; Della Ca’, N.; Sirianni, R.; Pezzi, V.; Gabriele, B. Catalytic Double Cyclization Process for Antitumor Agents against Breast Cancer Cell Lines. iScience 2018, 3, 279–288. [Google Scholar] [CrossRef]
- Acerbi, A.; Carfagna, C.; Costa, M.; Mancuso, R.; Gabriele, B.; Della Ca’, N. An Unprecedented Pd-Catalyzed Carbonylative Route to Fused Furo[3,4-b]indol-1-ones. Chem. Eur. J. 2018, 24, 4835–4840. [Google Scholar] [CrossRef]
- Veltri, L.; Giofrè, S.; Devo, P.; Romeo, R.; Dobbs, A.P.; Gabriele, B. A Palladium Iodide-Catalyzed Oxidative Aminocarbonylation-Heterocyclization Approach to Functionalized Benzimidazoimidazoles. J. Org. Chem. 2018, 83, 1680–1685. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; Veltri, L.; Russo, P.; Grasso, G.; Cuocci, C.; Romeo, R.; Gabriele, B. Palladium-Catalyzed Carbonylative Synthesis of Functionalized Benzimidazopyrimidinones. Synthesis 2018, 50, 267–277. [Google Scholar]
- Pancrazzi, F.; Motti, E.; Costa, M.; Mancuso, R.; Gabriele, B.; Della Ca’, N. (Z)-4-(carbomethoxymethylene)-2-(4-fluorophenyl)-4H-benzo[d][1,3]oxazine. Molbank 2017, 2017, M927. [Google Scholar] [CrossRef]
- Veltri, L.; Grasso, G.; Rizzi, R.; Mancuso, R.; Gabriele, B. Palladium-Catalyzed Carbonylative Multicomponent Synthesis of Functionalized Benzimidazothiazoles. Asian J. Org. Chem. 2016, 5, 560–567. [Google Scholar] [CrossRef]
- Mancuso, R.; Raut, D.S.; Marino, N.; De Luca, G.; Giordano, C.; Catalano, S.; Barone, I.; Andò, S.; Gabriele, B. A Palladium-Catalyzed Carbonylation Approach to Eight-Membered Lactam Derivatives with Antitumor Activity. Chem. Eur. J. 2016, 22, 3053–3064. [Google Scholar] [CrossRef]
- Veltri, L.; Mancuso, R.; Altomare, A.; Gabriele, B. Divergent Multicomponent Tandem Palladium-Catalyzed Aminocarbonylation-Cyclication Approached to Functionalized Imidazothiazinones and Imidazothiazoles. ChemCatChem 2015, 7, 2206–2213. [Google Scholar] [CrossRef]
- Gabriele, B.; Salerno, G.; Mancuso, R.; Costa, M. Efficient Synthesis of Ureas by Direct Palladium-Catalyzed Oxidative Carbonylation of Amines. J. Org. Chem. 2004, 69, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Didgikar, M.R.; Joshi, S.S.; Gupte, S.P.; Diwakar, M.M.; Deshpande, R.M.; Chaudhari, R.V. Oxidative carbonylation of amine using water-soluble palladium catalysts in biphasic media. J. Mol. Catal. A: Chem. 2011, 334, 20–28. [Google Scholar] [CrossRef]
- Kealey, S.; Husbands, S.M.; Bennacef, I.; Gee, A.D.; Passchier, J. Palladium-mediated oxidative reactions for the synthesis of 11C-radiolabelled ureas. J. Label. Compd. Radiopharm. 2014, 57, 202–208. [Google Scholar] [CrossRef]
- Troisi, L.; Granito, C.; Perrone, S.; Rosato, F. Synthesis of benzo-fused five and six-membered heterocycles by palladium-catalyzed cyclocarbonylation. Tetrahedron Lett. 2011, 52, 4330–4332. [Google Scholar] [CrossRef]
- Mizuno, T.; Nakai, T.; Mihara, M. Efficient Solvent-Free Synthesis of Urea Derivatives Using Selenium-Catalyzed Carbonylation of Amines with Carbon Monoxide. Synthesis 2010, 4251–4255. [Google Scholar] [CrossRef]
- The CO-O2 mixtures are potentially explosive over a large range of composition: the flammability range for CO in O2 is 16.7–93.5% at room temperature and it becomes even larger at higher temperatures. See: Bartish, C.M.; Drissel, G.M. Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed.; Grayson, M., Eckroth, D., Bushey, G.J., Campbell, L., Klingsberg, A., van Nes, L., Eds.; John Wiley & Sons: New York, NY, USA, 1978; Volume 4, p. 774. [Google Scholar]
- Casiello, M.; Iannone, F.; Cotugno, P.; Monopoli, A.; Cioffi, N.; Ciminale, F.; Trzeciak, A.M.; Nacci, A. Copper(II)-catalysed oxidative carbonylation to oxazolidinones, ureas and carbamates. J. Mol. Catal. A:Chem. 2015, 407, 8–14. [Google Scholar] [CrossRef]
- Wu, C.; Cheng, H.; Liu, R.; Wang, Q.; Hao, Y.; Yu, Y.; Zhao, F. Synthesis of urea derivatives from amines and CO2 in the absence of catalyst and solvent. Green Chem. 2010, 12, 1811–1816. [Google Scholar] [CrossRef]
- Paz, J.; Pérez-Balado, C.; Iglesias, B.; Muñoz, L. Carbon Dioxide as a Carbonylating Agent in the Synthesis of 2-Oxazolidinones, 2-Oxazinones, and Cyclic Ureas: Scope and Limitations. J. Org. Chem. 2010, 75, 3037–3046. [Google Scholar] [CrossRef]
- Kong, D.-L.; He, L.-N.; Wang, J.-Q. Synthesis of Urea Derivatives from CO2 and Amines Catalyzed by Polyethylene Glycol Supported Potassium Hydroxide without Dehydrating Agents. Synlett 2010, 1276–1280. [Google Scholar] [CrossRef]
- Streng, E.S.; Lee, D.S.; George, M.W.; Poliakoff, M. Continuous N-alkylation reactions of amino alcohols using γ-Al2O3 and supercritical CO2: unexpected formation of cyclic ureas and urethanes by reaction with CO2. Beilstein J. Org. Chem. 2017, 13, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Primo, A.; Aguado, E.; Garcia, H. CO2-Fixation on Aliphatic α,ω-Diamines fo Form Cyclic Ureas, Catalyzed by Ceria Nanoparticles that were Obtained by Templating with Alginate. ChemCatChem 2013, 5, 1010–1023. [Google Scholar] [CrossRef]
- Tamura, M.; Noro, K.; Honda, M.; Nakagawa, Y.; Tomishige, K. Highly efficient synthesis of cyclic ureas from CO2 and diamines by a pure CeO2 catalyst using 2-propanol solvent. Green Chem. 2013, 15, 1567–1577. [Google Scholar] [CrossRef]
- Xu, M.; Jupp, A.R.; Stephan, D.W. Stoichiometric Reactions of CO2 and Indium-Silylamides and Catalytic Synthesis of Ureas. Angew. Chem. Int. Ed. 2017, 56, 14277–14281. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, H.; Zhao, Y.; Chen, S.; Xu, J.; Hao, L.; Liu, Z. DBU-Based Ionic Liquid-Catalyzed Carbonylation of o-Phenylenediamines with CO2 to 2-Benzimidazolones under Solvent-Free Conditions. ACS Catal. 2013, 2, 2076–2082. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Yuan, G.; Hao, L.; Gao, X.; Yang, Z.; Yu, B.; Zhang, H.; Liu, Z. Azole-Anion-Based Aprotic Ionic Liquids: Functional Solvents for Atmospheric CO2 Transformation into Various Heterocyclic Compounds. Chem. Asian J. 2016, 11, 2735–2740. [Google Scholar] [CrossRef]
- Kimura, T.; Kamata, K.; Mizuno, N. A Bifunctional Tungstate Catalyst for Chemical Fixation of CO2 at Atmospheric Pressure. Angew. Chem., Int. Ed. 2012, 51, 6700–6703. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Kimura, T.; Sunaba, H.; Mizino, N. Scope of chemical fixation of carbon dioxide catalyzed by a bifunctional monomeric tungstate. Catal. Today 2014, 226, 160–166. [Google Scholar] [CrossRef]
- Zhu, Y.; Cornwall, R.G.; Du, H.; Zhao, B.; Shi, Y. Catalytic Diamination of Olefins via N–N Bond Activation. Acc. Chem. Res. 2014, 47, 3665–3678. [Google Scholar] [CrossRef] [PubMed]
- Muñiz, K.; Martínez, C. Development of Intramolecular Vicinal Diamination of Alkenes: From Palladium to Bromine Catalysis. J. Org. Chem. 2013, 78, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, R.M. Transition-Metal-Catalyzed Diamination of Olefins. Angew. Chem. Int. Ed. 2009, 48, 1190–1193. [Google Scholar] [CrossRef]
- Cardona, F.; Goti, A. Metal-catalysed 1,2-diamination reactions. Nat. Chem. 2009, 1, 269–275. [Google Scholar] [CrossRef]
- Streuff, J.; Hövelmann, C.H.; Nieger, M.; Muñiz, K. Palladium(II)-Catalyzed Intramolecular Diamination of Unfunctionalized Alkenes. J. Am. Chem. Soc. 2005, 127, 14586–14587. [Google Scholar] [CrossRef]
- Muñiz, K.; Hövelmann, C.H.; Streuff, J. Oxidative Diamination of Alkenes with Ureas as Nitrogen Sources: Mechanistic Pathways in the Presence of a High Oxidation State Palladium Catalyst. J. Am. Chem. Soc. 2008, 130, 763–773. [Google Scholar] [CrossRef]
- Muñiz, K.; Hövelmann, C.H.; Campos-Gómez, E.; Barluenga, J.; González, J.M.; Streuff, J.; Nieger, M. Intramolecular Diamination of Alkenes with Palladium(II)/Copper(II) Bromide and IPy2BF4: The Role of Halogenated Intermediates. Chem. Asian J. 2008, 3, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Muñiz, K.; Streuff, J.; Chávez, P.; Hövelmann, C.H. Synthesis of Diamino Carboxylic Esters by Palladium-Catalyzed Oxidative Intramolecular Diamination of Acrylates. Chem. Asian J. 2008, 3, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Muñiz, K.; Streuff, J.; Hövelmann, C.H.; Núñez, A. Exploring the Nickel-Catalyzed Oxidation of Alkenes: A Diamination by Sulfamide Transfer. Angew. Chem. Int. Ed. 2007, 46, 7125–7127. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, A.; Muñiz, K. Oxidative Interception of the Hydroamination Pathway: A Gold-Catalyzed Diamination of Alkenes. Chem. Eur. J. 2009, 15, 10563–10569. [Google Scholar] [CrossRef] [PubMed]
- Chávez, P.; Kirsch, J.; Hövelmann, C.H.; Streuff, J.; Martínez-Belmonte, M.; Escudero-Adán, E.C.; Martin, E.; Muñiz, K. Metal-free diamination of alkenes employing bromide catalysis. Chem. Sci. 2012, 3, 2375–2382. [Google Scholar] [CrossRef]
- Muñiz, K. Metal-free catalytic vicinal diamination of alkenes. Pure Appl. Chem. 2013, 85, 755–761. [Google Scholar] [CrossRef]
- Fu, S.; Yang, H.; Li, G.; Deng, Y.; Jiang, H.; Zeng, W. Copper(II)-Catalyzed Enantioselective Intramolecular Cyclization of N-Alkenylureas. Org. Lett. 2015, 17, 1018–1021. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Widenhoefer, R.A. Gold(I)-Catalyzed Intramolecular Dihydroamination of Allenes with N, N′-Disubstituted Ureas To Form Bicyclic Imidazolidin-2-ones. Org. Lett. 2009, 11, 2671–2674. [Google Scholar] [CrossRef] [PubMed]
- Bar, G.L.J.; Lloyd-Jones, G.C.; Booker-Milburn, K.I. Pd(II)-Catalyzed Intermolecular 1,2-Diamination of Conjugated Dienes. J. Am. Chem. Soc. 2005, 127, 7308–7309. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-S.; Fan, T.; Chen, S.-S.; Han, Z.-Y.; Gong, L.-Z. Pd(II)-Catalyzed Asymmetric Oxidative 1,2-Diamination of Conjugated Dienes with Ureas. Org. Lett. 2018, 20, 2485–2489. [Google Scholar] [CrossRef]
- Du, H.; Yuan, W.; Zhao, B.; Shi, Y. Catalytic Asymmetric Diamination of Conjugated Dienes and Triene. J. Am. Chem. Soc. 2007, 129, 11688–11689. [Google Scholar] [CrossRef]
- Du, H.; Zhao, B.; Shi, Y. A Facile Pd(0)-Catalyzed Regio- and Stereoselective Diamination of Conjugated Dienes and Trienes. J. Am. Chem. Soc. 2007, 129, 762–763. [Google Scholar] [CrossRef]
- Zhao, B.; Du, H.; Cui, S.; Shi, Y. Synthetic and Mechanistic Studies on Pd(0)-Catalyzed Diamination of Conjugated Dienes. J. Am. Chem. Soc. 2010, 132, 3523–3532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Du, H.; Shi, Y. Diamination of Conjugated Dienes and Trienes Catalyzed by N-Heterocyclic Carbene−Pd(0) Complexes. J. Org. Chem. 2007, 72, 7038–7041. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shi, Y. Chiral N-Heterocyclic Carbene−Pd(0)-Catalyzed Asymmetric Diamination of Conjugated Dienes and Triene. J. Org. Chem. 2008, 73, 749–751. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yuan, W.; Zhao, B.; Shi, Y. A Pd(0)-Catalyzed Diamination of Terminal Olefins at Allylic and Homoallylic Carbons via Formal C−H Activation under Solvent-Free Conditions. J. Am. Chem. Soc. 2007, 129, 7496–7497. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhao, B.; Shi, Y. Catalytic Asymmetric Allylic and Homoallylic Diamination of Terminal Olefins via Formal C−H Activation. J. Am. Chem. Soc. 2008, 130, 8590–8591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, R.; Zhao, B.; Shi, Y. Synthesis of (+)-CP-99,994 via Pd(0)-Catalyzed Asymmetric Allylic and Homoallylic C−H Diamination of Terminal Olefin. J. Org. Chem. 2009, 74, 7577–7580. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, T.A.; Wang, Q.; Zhu, Y.; Zheng, H.; Peng, X.; Cornwall, R.G.; Shi, Y. Pd(0)-Catalyzed Sequential C–N Bond Formation via Allylic and Aromatic C–H Amination of α-Methylstyrenes with Diaziridinone. Org. Lett. 2013, 15, 4210–4213. [Google Scholar] [CrossRef] [Green Version]
- Yuan, W.; Du, H.; Zhao, B.; Shi, Y. A Mild Cu(I)-Catalyzed Regioselective Diamination of Conjugated Dienes. Org. Lett. 2007, 9, 2589–2591. [Google Scholar] [CrossRef]
- Du, H.; Zhao, B.; Yuan, W.; Shi, Y. Cu(I)-Catalyzed Asymmetric Diamination of Conjugated Dienes. Org. Lett. 2008, 10, 4231–4234. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Peng, X.; Cui, S.; Shi, Y. Cu(I)-Catalyzed Regioselective Diamination of Conjugated Dienes via Dual Mechanistic Pathways. J. Am. Chem. Soc. 2010, 132, 11009–11011. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Peng, X.; Zhu, Y.; Ramirez, T.A.; Cornwall, R.G.; Shi, Y. Cu(I)-Catalyzed Diamination of Conjugated Dienes. Complementary Regioselectivity from Two Distinct Mechanistic Pathways Involving Cu(II) and Cu(III) Species. J. Am. Chem. Soc. 2011, 133, 20890–20900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Y.; Zhao, B.; Shi, Y. Cu(I)-Catalyzed Diamination of Disubstituted Terminal Olefins: An Approach to Potent NK1 Antagonist. Org. Lett. 2009, 11, 2365–2368. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Saunthwal, R.K.; Verma, A.K. Base-Mediated Hydroamination of Alkynes. Acc. Chem. Res. 2017, 50, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Arndt, M.; Gooßen, K.; Heydt, H.; Gooßen, L.J. Late Transition Metal-Catalyzed Hydroamination and Hydroamidation. Chem. Rev. 2015, 115, 2596–2697. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.E.; Hultzsch, K.C.; Yus, M.; Foubelo, F.; Tada, M. Hydroamination: Direct Addition of Amines to Alkenes and Alkynes. Chem. Rev. 2008, 108, 3795–3892. [Google Scholar] [CrossRef] [PubMed]
- Pohlki, F.; Doye, S. The Catalytic Hydroamination of Alkynes. Chem. Soc. Rev. 2003, 32, 104–114. [Google Scholar] [CrossRef]
- Johns, A.M.; Sakai, N.; Ridder, A.; Hartwig, J.F. Direct Measurement of the Thermodynamics of Vinylarene Hydroamination. J. Am. Chem. Soc. 2006, 128, 9306–9307. [Google Scholar] [CrossRef]
- Schlummer, B.; Hartwig, J.F. Brønsted Acid-Catalyzed Intramolecular Hydroamination of Protected Alkenylamines. Synthesis of Pyrrolidines and Piperidines. Org. Lett. 2002, 4, 1471–1474. [Google Scholar] [CrossRef]
- Dion, I.; Beauchemin, A.M. Asymmetric Brønsted Acid Catalysis Enabling Hydroaminations of Dienes and Allenes. Angew. Chem. Int. Ed. 2011, 50, 8233–8235. [Google Scholar] [CrossRef]
- Rodriguez, A.L.; Koradin, C.; Dohle, W.; Knochel, P. Versatile Indole Synthesis by a 5-Endo-Dig Cyclization Mediated by Potassium or Cesium Bases. Angew. Chem. Int. Ed. 2000, 39, 2488–2490. [Google Scholar] [CrossRef]
- Seayad, J.; Tillack, A.; Hartung, C.G.; Beller, M. Base-Catalyzed Hydroamination of Olefins: An Environmentally Friendly Route to Amines. Adv. Synth. Catal. 2002, 344, 795–813. [Google Scholar] [CrossRef]
- Herrero, M.T.; de Sarralde, J.D.; SanMartin, R.; Bravo, L.; Domínguez, E. Cesium Carbonate-Promoted Hydroamidation of Alkynes: Enamides, Indoles and the Effect of Iron(III) Chloride. Adv. Synth. Catal. 2012, 354, 3054–3064. [Google Scholar] [CrossRef]
- Gooßen, L.J.; Rauhaus, J.E.; Deng, G. Ru-Catalyzed Anti-Markovnikov Addition of Amides to Alkynes: A Regio- and Stereoselective Synthesis of Enamides. Angew. Chem. Int. Ed. 2005, 44, 4042–4045. [Google Scholar] [CrossRef] [PubMed]
- LaLonde, R.L.; Sherry, B.D.; Kang, E.J.; Toste, F.D. Gold(I)-Catalyzed Enantioselective Intramolecular Hydroamination of Allenes. J. Am. Chem. Soc. 2007, 129, 2452–2453. [Google Scholar] [CrossRef] [PubMed]
- Pflästerer, D.; Dolbundalchok, P.; Rafique, S.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. On the Gold-Catalyzed Intramolecular 7-Exo-Trig Hydroamination of Allenes. Adv. Synth. Catal. 2013, 355, 1383–1393. [Google Scholar] [CrossRef]
- Zhang, Z.; Lee, S.D.; Widenhoefer, R.A. Intermolecular Hydroamination of Ethylene and 1-Alkenes with Cyclic Ureas Catalyzed by Achiral and Chiral Gold(I) Complexes. J. Am. Chem. Soc. 2009, 131, 5372–5373. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Buchwald, S.L. Rhodium-Catalyzed Asymmetric Intramolecular Hydroamination of Unactivated Alkenes. Angew. Chem. 2010, 122, 574–577. [Google Scholar] [CrossRef]
- Hannedouche, J.; Schulz, E. Hydroamination and Hydroaminoalkylation of Alkenes by Group 3–5 Elements: Recent Developments and Comparison with Late Transition Metals. Organomet. 2018. [Google Scholar] [CrossRef]
- Gilmore, K.; Mohamed, R.K.; Alabugin, I.V. The Baldwin Rules: Revised and Extended: Baldwin: Revised, Extended. Wiley Interdiscip. Rev. Comp. Mol. Sci. 2016, 6, 487–514. [Google Scholar] [CrossRef]
- Peshkov, V.A.; Pereshivko, O.P.; Sharma, S.; Meganathan, T.; Parmar, V.S.; Ermolat’ev, D.S.; Van der Eycken, E.V. Tetrasubstituted 2-Imidazolones via Ag(I)-Catalyzed Cycloisomerization of Propargylic Ureas. J. Org. Chem. 2011, 76, 5867–5872. [Google Scholar] [CrossRef]
- Pereshivko, O.P.; Peshkov, V.A.; Jacobs, J.; Meervelt, L.V.; Van der Eycken, E.V. Cationic Gold-and Silver-Catalyzed Cycloisomerizations of Propargylic Ureas: A Selective Entry to Oxazolidin-2-Imines and Imidazolidin-2-Ones. Adv. Synth. Catal. 2013, 355, 781–789. [Google Scholar] [CrossRef]
- La-Venia, A.; Medran, N.S.; Krchňák, V.; Testero, S.A. Synthesis of a Small Library of Imidazolidin-2-Ones Using Gold Catalysis on Solid Phase. ACS Comb. Sci. 2016, 18, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Alamsetti, S.K.; Persson, A.K.Å.; Bäckvall, J.-E. Palladium-Catalyzed Intramolecular Hydroamination of Propargylic Carbamates and Carbamothioates. Org. Lett. 2014, 16, 1434–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Shen, M.; Zhang, Y.; Li, G.; Khodabocus, A.; Rodriguez, S.; Qu, B.; Farina, V.; Senanayake, C.H.; Lu, B.Z. A New Entry to Polycyclic Indole Skeletons via Palladium-Catalyzed Intramolecular Heteroannulation. Org. Lett. 2006, 8, 3573–3575. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Li, G.; Roschangar, F.; Farina, V.; Senanayake, C.H.; Lu, B.Z. A Novel One-Pot, Two-Step Synthesis of Polycyclic Indoles via Tandem Intramolecular Hydroamidation/Palladium-Catalyzed Annulation. Adv. Synth. Catal. 2010, 352, 2667–2671. [Google Scholar] [CrossRef]
- Proulx, C.; Lubell, W.D. N-Amino-Imidazolin-2-One Peptide Mimic Synthesis and Conformational Analysis. Org. Lett. 2012, 14, 4552–4555. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Yerande, R.; Wakchaure, P.B.; Yerande, S.G.; Dethe, D.H. Base-Mediated Hydroamination of Propargylamine: A Regioselective Intramolecular 5-Exo-Dig Cycloisomerization En Route to Imidazole-2-Thione. Org. Lett. 2014, 16, 5788–5791. [Google Scholar] [CrossRef] [PubMed]
- Huguenot, F.; Delalande, C.; Vidal, M. Metal-Free 5-Exo-Dig Cyclization of Propargyl Urea Using TBAF. Tetrahedron Lett. 2014, 55, 4632–4635. [Google Scholar] [CrossRef]
- Arrowsmith, M.; Shepherd, W.M.S.; Hill, M.S.; Kociok-Köhn, G. Alkaline Earth Catalysis for the 100% Atom-Efficient Three Component Assembly of Imidazolidin-2-Ones. Chem. Commun. 2014, 50, 12676–12679. [Google Scholar] [CrossRef]
- Martínez, A.; Moreno-Blázquez, S.; Rodríguez-Diéguez, A.; Ramos, A.; Fernández-Galán, R.; Antiñolo, A.; Carrillo-Hermosilla, F. Simple ZnEt2 as a Catalyst in Carbodiimide Hydroalkynylation: Structural and Mechanistic Studies. Dalton Trans. 2017, 46, 12923–12934. [Google Scholar] [CrossRef]
- Fritz, J.A.; Nakhla, J.S.; Wolfe, J.P. A New Synthesis of Imidazolidin-2-Ones via Pd-Catalyzed Carboamination of N-Allylureas. Org. Lett. 2006, 8, 2531–2534. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.A.; Wolfe, J.P. Stereoselective Synthesis of Imidazolidin-2-Ones via Pd-Catalyzed Alkene Carboamination. Scope and Limitations. Tetrahedron 2008, 64, 6838–6852. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.A.; Wolfe, J.P. Synthesis of Enantiomerically Enriched Imidazolidin-2-Ones through Asymmetric Palladium-Catalyzed Alkene Carboamination Reactions. Angew. Chem. Int. Ed. 2012, 51, 9886–9890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, H.; Pan, R.; Lin, X. Synthesis and Application of a New Chiral Monodentate Spiro Phosphoramidite Ligand Based on Hexamethyl-1,1′-Spirobiindane Backbone in Asymmetric Hydroamination/Arylation of Alkenes. Org. Biomol. Chem. 2018, 16, 6183–6186. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.J.; Kirsch, J.K.; Wolfe, J.P. Pd-Catalyzed Alkene Diamination Reactions of Nitrogen Electrophiles: Synthesis of Cyclic Guanidines and Ureas Bearing Dialkylaminomethyl Groups. Org. Lett. 2018, 20, 3513–3517. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, F.C.; Turnpenny, B.W.; Chemler, S.R. Copper-Promoted and Copper-Catalyzed Intermolecular Alkene Diamination. Angew. Chem. 2010, 122, 6509–6512. [Google Scholar] [CrossRef]
- Zabawa, T.P.; Chemler, S.R. Copper(II) Carboxylate Promoted Intramolecular Diamination of Terminal Alkenes: Improved Reaction Conditions and Expanded Substrate Scope. Org. Lett. 2007, 9, 2035–2038. [Google Scholar] [CrossRef]
- Xiong, P.; Xu, F.; Qian, X.-Y.; Yohannes, Y.; Song, J.; Lu, X.; Xu, H.-C. Copper-Catalyzed Intramolecular Oxidative Amination of Unactivated Internal Alkenes. Chem. Eur. J. 2016, 22, 4379–4383. [Google Scholar] [CrossRef]
- Li, H.; Song, F.; Widenhoefer, R.A. Gold(I)-Catalyzed Intramolecular Hydroamination of N-Allylic N′-Arylureas to Form Imidazolidin-2-Ones. Adv. Synth. Catal. 2011, 353, 955–962. [Google Scholar] [CrossRef]
- Zhang, G.; Luo, Y.; Wang, Y.; Zhang, L. Combining Gold(I)/Gold(III) Catalysis and C-H Functionalization: A Formal Intramolecular [3+2] Annulation towards Tricyclic Indolines and Mechanistic Studies. Angew. Chem. Int. Ed. 2011, 50, 4450–4454. [Google Scholar] [CrossRef]
- Manick, A.-D.; Aubert, S.; Yalcouye, B.; Prangé, T.; Berhal, F.; Prestat, G. Access to Functionalized Imidazolidin-2-One Derivatives by Iron-Catalyzed Oxyamination of Alkenes. Chem. Eur. J. 2018, 24, 11485–11492. [Google Scholar] [CrossRef] [PubMed]
- Baeg, J.-O.; Alper, H. Novel Palladium(II)-Catalyzed Cyclization of Aziridines and Sulfur Diimides. J. Am. Chem. Soc. 1994, 116, 1220–1224. [Google Scholar] [CrossRef]
- Butler, D.C.D.; Inman, G.A.; Alper, H. Room Temperature Ring-Opening Cyclization Reactions of 2-Vinylaziridines with Isocyanates, Carbodiimides, and Isothiocyanates Catalyzed by [Pd(OAc)2]/PPh3. J. Org. Chem. 2000, 65, 5887–5890. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Fandrick, D.R. Dynamic Kinetic Asymmetric Cycloadditions of Isocyanates to Vinylaziridines. J. Am. Chem. Soc. 2003, 125, 11836–11837. [Google Scholar] [CrossRef]
- Dong, C.; Alper, H. CeCl3 promoted asymmetric cycloaddition of isocyanates with 2-vinylaziridines. Tetrahedron: Asymmetry 2004, 15, 1537–1540. [Google Scholar] [CrossRef]
- Okano, A.; Oishi, S.; Tanaka, T.; Fujii, N.; Ohno, H. Construction of Linked Nitrogen Heterocycles by Palladium(0)-Catalyzed Intramolecular Domino Cyclization of 2-Alkynylaziridines Bearing a 2-Aminoethyl Group via Ring Expansion with Isocyanate. J. Org. Chem. 2010, 75, 3396–3400. [Google Scholar] [CrossRef] [PubMed]
- Munegumi, T.; Azumaya, I.; Kato, T.; Masu, H.; Saito, S. [3+2] Cross-Coupling Reactions of Aziridines with Isocyanates Catalyzed by Nickel(II) Iodide. Org. Lett. 2006, 8, 379–382. [Google Scholar] [CrossRef]
- Zhang, K.; Chopade, P.R.; Louie, J. Coupling of vinyl aziridines and phenyl isocyanate. Tetrahedron Lett. 2008, 49, 4306–4309. [Google Scholar] [CrossRef] [Green Version]
- Nadir, U.K.; Krishna, R.V.; Singh, A. A new and facile route for the synthesis of chiral 1,2-diamines and 2,3-diamino acids. Tetrahedron Lett. 2005, 46, 479–482. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, Y.-W.; Hahm, H.S.; Jang, J.W.; Lee, W.K.; Ha, H.-J. Lewis acid-catalyzed stereospecific ring expansion of aziridine-2-carboxylates to imidazolidin-2-ones. Chem. Commun. 2005, 3062–3064. [Google Scholar] [CrossRef]
- Eum, H.-S.; Lee, Y.-N.; Kim, S.-M.; Baek, A.-Y.; Son, M.-K.; Lee, K.-W.; Ko, S.-W.; Kim, S.-H.; Yun, S.-Y.; Lee, W.-K.; et al. Synthesis of Substituted Imidazolidin-2-ones as Aminoacyl-tRNA Synthase Inhibitors. Bull. Korean Chem. Soc. 2010, 31, 611–614. [Google Scholar] [CrossRef] [Green Version]
- Yoon, D.-H.; Ha, H.-J.; Kim, B.C.; Lee, W.K. Conjugate addition of amines to chiral 3-aziridin-2-yl-acrylates. Tetrahedron Lett. 2010, 51, 2181–2183. [Google Scholar] [CrossRef]
- Tabarki, M.A.; Besbes, R. Ring expansion of aziridine-2-carboxylates. An efficient entry to imidazolidin-2-ones and oxazolidin-2-imines. Tetrahedron Lett. 2015, 56, 1837–1839. [Google Scholar] [CrossRef]
- Hinds, E.M.; Wolfe, J.P. A Cross-Metathesis/Aza-Michael Reaction Strategy for the Synthesis of Cyclic and Bicyclic Ureas. J. Org. Chem. 2018, 83, 10668–10676. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, A.; Kamata, Y.; Terada, M. Synthesis of Enantioenriched γ-Amino-α,β-Unsaturated Esters Utilizing Palladium-Catalyzed Rearrangement of Allylic Carbamates for Direct Application to Formal [3 + 2] Cycloaddition. Org. Lett. 2017, 19, 1682–1685. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Dai, C.; Huang, Y. DBU-Catalyzed Desymmetrization of Cyclohexadienones: Access to Vicinal Diamine-Containing Heterocycles. Org. Lett. 2018, 20, 5006–5009. [Google Scholar] [CrossRef] [PubMed]
- Kutovaya, I.V.; Shmatova, O.I.; Tkachuk, V.M.; Melnichenko, N.V.; Vovk, M.V.; Nenajdenko, V.G. Aza-Henry Reaction with CF3 -Ketimines: An Efficient Approach to Trifluoromethylated β-Nitroamines, 1,2-Diamines, α- Aminooximes, and Imidazolidinones: Aza-Henry Reaction with CF 3 -Ketimines. Eur. J. Org. Chem. 2015, 2015, 6749–6761. [Google Scholar] [CrossRef]
- Wiebe, A.; Gieshoff, T.; Möhle, S.; Rodrigo, E.; Zirbes, M.; Waldvogel, S.R. Electrifying Organic Synthesis. Angew. Chem. Int. Ed. 2018, 57, 5594–5619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, P.; Xu, H.-H.; Xu, H.-C. Metal- and Reagent-Free Intramolecular Oxidative Amination of Tri- and Tetrasubstituted Alkenes. J. Am. Chem. Soc. 2017, 139, 2956–2959. [Google Scholar] [CrossRef]
- Ahmed, N.; Khatoon, S. Facile Electrochemical Intramolecular Amination of Urea-Tethered Terminal Alkenes for the Synthesis of Cyclic Ureas. ChemistryOpen 2018, 7, 576–582. [Google Scholar] [CrossRef]
- Strambeanu, I.I.; White, M.C. Catalyst-Controlled C–O versus C–N Allylic Functionalization of Terminal Olefins. J. Am. Chem. Soc. 2013, 135, 12032–12037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Shuler, S.A.; Watson, D.A. Synthesis of N−H Bearing Imidazolidinones and Dihydroimidazolones Using Aza-Heck Cyclizations. Angew. Chem. Int. Ed. 2018, 57, 12081–12085. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Gao, C.; Song, Z.; Yang, H.; Fu, H. Metal-Free Oxidative C-H Amidation of N, N′-Diarylureas with PhI(OAc)2: Synthesis of Benzimidazol-2-One Derivatives: Metal-Free Oxidative C-H Amidation of N, N′-Diarylureas. Eur. J. Org. Chem. 2015, 5869–5875. [Google Scholar] [CrossRef]
- Zeng, R.; Chen, P.; Dong, G. Efficient Benzimidazolidinone Synthesis via Rhodium-Catalyzed Double-Decarbonylative C–C Activation/Cycloaddition between Isatins and Isocyanates. ACS Catal. 2016, 6, 969–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchegiani, M.; Nodari, M.; Tansini, F.; Massera, C.; Mancuso, R.; Gabriele, B.; Costa, M.; Della Ca’, N. Urea derivatives from carbon dioxide and amines by guanidine catalysis: Easy access to imidazolidin-2-ones under solvent-free conditions. J. CO2 Util. 2017, 21, 553–561. [Google Scholar] [CrossRef]







































































© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casnati, A.; Motti, E.; Mancuso, R.; Gabriele, B.; Della Ca’, N. Recent Advances in the Catalytic Synthesis of Imidazolidin-2-ones and Benzimidazolidin-2-ones. Catalysts 2019, 9, 28. https://doi.org/10.3390/catal9010028
Casnati A, Motti E, Mancuso R, Gabriele B, Della Ca’ N. Recent Advances in the Catalytic Synthesis of Imidazolidin-2-ones and Benzimidazolidin-2-ones. Catalysts. 2019; 9(1):28. https://doi.org/10.3390/catal9010028
Chicago/Turabian StyleCasnati, Alessandra, Elena Motti, Raffaella Mancuso, Bartolo Gabriele, and Nicola Della Ca’. 2019. "Recent Advances in the Catalytic Synthesis of Imidazolidin-2-ones and Benzimidazolidin-2-ones" Catalysts 9, no. 1: 28. https://doi.org/10.3390/catal9010028
APA StyleCasnati, A., Motti, E., Mancuso, R., Gabriele, B., & Della Ca’, N. (2019). Recent Advances in the Catalytic Synthesis of Imidazolidin-2-ones and Benzimidazolidin-2-ones. Catalysts, 9(1), 28. https://doi.org/10.3390/catal9010028