The Role of Iodine Catalyst in the Synthesis of 22-Carbon Tricarboxylic Acid and Its Ester: A Case Study
Abstract
:1. Introduction
2. Results and Discussion
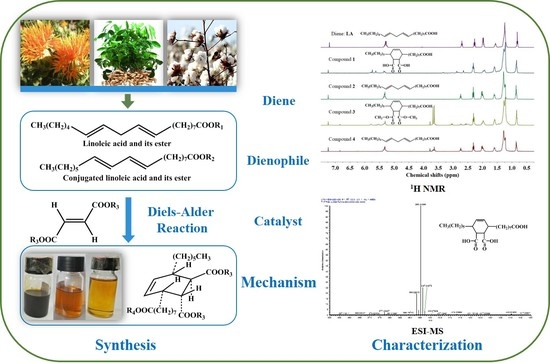
2.1. Synthesis and Characterization
2.2. The Role of Iodine in Diels–Alder Reaction
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Methyl Linoleate (ML)
3.3. Synthesis of 22-Carbon Tricarboxylic Acid (C22TA) and 22-Carbon Tricarboxylic Ester (C22TAE)
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Table of Contents

References
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels Production by Biomass Gasification: A Review. Energies 2018, 11, 811. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Biresaw, G.; Sharma, B.K.; Erhan, S.Z. Friction Behavior of Some Seed Oils: Biobased Lubricant Applications. Ind. Eng. Chem. Res. 2006, 45, 3735–3740. [Google Scholar] [CrossRef]
- Salimon, J.; Abdullah, B.M.; Yusop, R.M.; Salih, N. Synthesis, reactivity and application studies for different biolubricants. Chem. Cent. J. 2014, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Visentini, F.F.; Perez, A.A.; Santiago, L.G. Self-assembled nanoparticles from heat treated ovalbumin as nanocarriers for polyunsaturated fatty acids. Food Hydrocoll. 2019, 93, 242–252. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef]
- Fox, N.J.; Stachowiak, G.W. Vegetable oil-based lubricants—A review of oxidation. Tribol. Int. 2007, 40, 1035–1046. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, P.; Zhang, J.; Li, S.; Li, M.; Xia, J.; Zhou, Y. Preparation of biobased epoxies using tung oil fatty acid-derived C21 diacid and C22 triacid and study of epoxy properties. Green Chem. 2013, 15, 2466–2475. [Google Scholar] [CrossRef]
- Vijayalakshmi, P.; Subbarao, R.; Lakshminarayana, G. Preparation and surface-active properties of the sodium soaps, mono- and diethanolamides and diol and triol sulfates of cycloaliphatic C21 Di- and C22 Tricarboxylic acids. J. Am. Oil Chem. Soc. 1991, 68, 133–137. [Google Scholar] [CrossRef]
- Fischer, E.R.; Boyd, P.G. New Water Soluble C22 Tricarboxylic Acid Amine Salts and Their Use as a Water Soluble Corrosion Inhibitors. E.P. Patent No. 0,913,384, 6 May 1999. [Google Scholar]
- Watanabe, S.; Fujita, T.; Fukuda, S.; Hirano, K.; Sakamoto, M. Characteristic properties as cutting fluid additives of the products from the reaction of unsaturated fatty acids with maleic anhydride. Mater. Chem. Phys. 1986, 15, 89–96. [Google Scholar] [CrossRef]
- Powers, J.R.; Pleasant, M.; Miller, E.C.; Charleston, S.C. C22 -Cycloaliphatic Tricarboxylic Fatty Acid Soaps. U.S. Patent No. 4,081,462, 28 March 1978. [Google Scholar]
- Chintareddy, V.R.; Oshel, R.E.; Doll, K.M.; Yu, Z.; Wu, W.; Zhang, G.; Verkade, J.G. Investigation of Conjugated Soybean Oil as Drying Oils and CLA Sources. J. Am. Oil Chem. Soc. 2012, 89, 1749–1762. [Google Scholar] [CrossRef]
- Jain, V.P.; Proctor, A.; Lall, R. Pilot-Scale Production of Conjugated Linoleic Acid-Rich Soy Oil by Photoirradiation. J. Food Sci. 2008, 73, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Kadamne, J.V.; Castrodale, C.L.; Proctor, A. Measurement of Conjugated Linoleic Acid (CLA) in CLA-Rich Potato Chips by ATR-FTIR Spectroscopy. J. Agric. Food Chem. 2011, 59, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Gangidi, R.R.; Proctor, A. Photochemical production of conjugated linoleic acid from soybean oil. Lipids 2004, 39, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.P.; Proctor, A. Photocatalytic Production and Processing of Conjugated Linoleic Acid-Rich Soy Oil. J. Agric. Food Chem. 2006, 54, 5590–5596. [Google Scholar] [CrossRef] [PubMed]
- Tokle, T.; Jain, V.P.; Proctor, A. Effect of Minor Oil Constituents on Soy Oil Conjugated Linoleic Acid Production. J. Agric. Food Chem. 2009, 57, 8989–8997. [Google Scholar] [CrossRef]
- Balakrishna, R.S.; Murthy, B.G.K.; Aggarwal, J.S. Diels-Alder adducts from safflower oil fatty acids. J. Am. Oil Chem. Soc. 1971, 48, 689–692. [Google Scholar] [CrossRef]
- Miller, W.R.; Bell, E.W.; Cowan, J.C.; Teeter, H.M. Reactions of dienophiles with vegetable oils. I. Reactions of maleic esters with sulfur dioxide catalyst. J. Am. Oil Chem. Soc. 1959, 36, 394–397. [Google Scholar] [CrossRef]
- Danzig, M.J.; O’Donnell, J.L.; Bell, E.W.; Cowan, J.C.; Teeter, H.M. Reactions of conjugated fatty acids. V. Preparation and properties of diels-alder adducts and their esters fromtrans,trans-conjugated fatty acids derived from soybean oil. J. Am. Oil Chem. Soc. 1957, 34, 136–138. [Google Scholar] [CrossRef]
- Bickford, W.G.; DuPré, E.F.; Mack, C.H.; O’Connor, R.T. The infrared spectra and the structural relationships between alpha- and beta-eleostearic acids and their maleic anhydride adducts. J. Am. Oil Chem. Soc. 1953, 30, 376–381. [Google Scholar] [CrossRef]
- Teeter, H.M.; O’Donnell, J.L.; Schneider, W.J.; Gast, L.E.; Danzig, M.J. Reactions of Conjugated Fatty Acids. IV. Diels-Alder Adducts of 9,11-Octadecadienoic. Acid. J. Org. Chem. 1957, 22, 512–514. [Google Scholar] [CrossRef]
- Philippaerts, A.; Goossens, S.; Jacobs, P.A.; Sels, B.F. Catalytic production of conjugated fatty acids and oils. ChemSusChem 2011, 4, 684–702. [Google Scholar] [CrossRef] [PubMed]
- Cihelkova, K.; Schieber, A.; Lopes-Lutz, D.; Hradkova, I.; Kyselka, J.; Filip, V. Quantitative and qualitative analysis of high molecular compounds in vegetable oils formed under high temperatures in the absence of oxygen. Eur. Food Res. Technol. 2013, 237, 71–81. [Google Scholar] [CrossRef]
- Ward, B.E.; Charleston, S.C. Selective Reaction of Fatty Acids and Their Separation. U.S. Patent No. 3,753,968, 21 August 1973. [Google Scholar]
- Benson, S.W.; Bose, A.N. The Iodine-catalyzed, Positional Isomerization of Olefins. A New Tool for the Precise Measurement of Thermodynamic Data. J. Am. Chem. Soc. 1963, 85, 1385–1387. [Google Scholar] [CrossRef]
- Egger, K.W.; Benson, S.W. Iodine and Nitric Oxide Catalyzed Isomerization of Olefins. V. Kinetics of the Geometrical Isomerization of 1,3-Pentadiene, a Check on the Rate of Rotation about Single Bonds, and the Allylic Resonance Energy. J. Am. Chem. Soc. 1965, 87, 3314–3319. [Google Scholar] [CrossRef]
- Afarin, M.; Alemzadeh, I.; Yazdi, Z.K. Conjugated Linoleic Acid Production and Optimization Via Catalytic Reaction Method Using Safflower Oil. Int. J. Eng. 2018, 31, 1166–1171. [Google Scholar] [CrossRef]
- Silva-Ramirez, A.S.; Rocha-Uribe, A.; Gonzalez-Chavez, M.M.; Gonzalez, C. Synthesis of conjugated linoleic acid by microwave-assisted alkali isomerization using propylene glycol as solvent. Eur. J. Lipid Sci. Technol. 2017, 119, 9. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Li, X.; Du, K.F.; Yao, S.; Song, H. Conjugated Linoleic Acid Production by Alkali Isomerization of Linoleic Acid from Idesia polycarpa Maxim. var. vestita Diels Oil. Asian J. Chem. 2013, 25, 3744–3748. [Google Scholar] [CrossRef]
- Falkenburg, L.B.; DeJong, W.M.; Handke, D.P.; Radlove, S.B. Isomerization of drying and semi-drying oils: The use of anthraquinone as a conjugation catalyst. J. Am. Oil Chem. Soc. 1948, 25, 237–243. [Google Scholar] [CrossRef]
- Chen, C.A.; Lu, W.; Sih, C.J. Synthesis of 9Z,11E-octadecadienoic and 10E,12Z-octadecadienoic acids, the major components of conjugated linoleic acid. Lipids 1999, 34, 879–884. [Google Scholar] [CrossRef]
- Guerrero-Corella, A.; Asenjo-Pascual, J.; Pawar, T.J.; Díaz-Tendero, S.; Martín-Sómer, A.; Gómez, C.V.; Belmonte-Vázquez, J.L.; Ramírez-Ornelas, D.E.; Peña-Cabrera, E.; Fraile, A.; et al. BODIPY as electron withdrawing group for the activation of double bonds in asymmetric cycloaddition reactions. Chem. Sci. 2019, 10, 4346–4351. [Google Scholar] [CrossRef]
- Houk, K.N.; Sims, J.; Duke, R.E.; Strozier, R.W.; George, J.K. Frontier molecular orbitals of 1,3 dipoles and dipolarophiles. J. Am. Chem. Soc. 1973, 95, 7287–7301. [Google Scholar] [CrossRef]
- Sauer, J.; Sustmann, R. Mechanistic Aspects of Diels-Alder Reactions: A Critical Survey. Angew. Chem. Int. Ed. 1980, 19, 779–807. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H. Enhancement of efficiency in luminescent polymer by incorporation of conjugated 1,3,4-oxadiazole side chains as hole-blocker/electron-transporter. Synth. Met. 2004, 143, 13–19. [Google Scholar] [CrossRef]
- Fringuelli, F.; Taticchi, A. Diels–Alder Reaction: General Remarks. In The Diels–Alder Reaction; John Wiley and Sons: New York, NY, USA, 2002; pp. 1–28. [Google Scholar] [CrossRef]
- Fringuelli, F.; Minuti, L.; Pizzo, F.; Taticchi, A. Reactivity and Selectivity in Lewis-Acid-Catalyzed Diels-Alder Reactions of 2-Cyclohexenones. ChemInform; Munksgaard: Copenhagen, Denmark, 1993; p. 24. [Google Scholar] [CrossRef]
- Zeng, X.; Yu, S.; Sun, R. Effect of functionalized multiwall carbon nanotubes on the curing kinetics and reaction mechanism of bismaleimide–triazine. J. Therm. Anal. Calorim. 2013, 114, 387–395. [Google Scholar] [CrossRef]






| Entry | Reaction Conditions | Compounds | |||
|---|---|---|---|---|---|
| Dienes (1 mol) | Dienophiles (1.1 mol) | Catalyst (0.3 wt.%) 1 | Category | Yield (%) | |
| 1 | LA | FA | Iodine | 22-Carbon tricarboxylic acid (C22TA) | 71.9 |
| 2 | LA | FA | / | / | / |
| 3 | LA | DF | Iodine | 22-Carbon tricarboxylic acid dimethyl ester (C22TADME) | 81.3 |
| 4 | LA | DF | / | / | / |
| 5 | ML | FA | Iodine | 22-Carbon tricarboxylic acid monomethyl ester (C22TAMME) | 65.4 |
| 6 | ML | FA | / | / | / |
| 7 | ML | DF | Iodine | 22-Carbon tricarboxylic acid trimethyl ester (C22TATME) | 76.3 |
| 8 | ML | DF | / | / | / |
| 9 | CLA | DF | Iodine | 22-Carbon tricarboxylic acid dimethyl ester (C22TADME) | 82.0 |
| 10 | CLA | DF | / | 79.4 | |
| 11 | CLAEE | FA | Iodine | 22-Carbon tricarboxylic acid monoethyl ester (C22TAMEE) | 74.4 |
| 12 | CLAEE | FA | / | 71.4 | |
| 13 | CLAEE | DF | Iodine | 22-Carbon tricarboxylic acid dimethyl monoethyl ester (C22TADMMEE) | 83.3 |
| 14 | CLAEE | DF | / | 77.5 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, Y.; Wang, L.; Zan, X.; Zhang, L. The Role of Iodine Catalyst in the Synthesis of 22-Carbon Tricarboxylic Acid and Its Ester: A Case Study. Catalysts 2019, 9, 972. https://doi.org/10.3390/catal9120972
Liu Y, Zhang Y, Wang L, Zan X, Zhang L. The Role of Iodine Catalyst in the Synthesis of 22-Carbon Tricarboxylic Acid and Its Ester: A Case Study. Catalysts. 2019; 9(12):972. https://doi.org/10.3390/catal9120972
Chicago/Turabian StyleLiu, Yanxia, Yagang Zhang, Lulu Wang, Xingjie Zan, and Letao Zhang. 2019. "The Role of Iodine Catalyst in the Synthesis of 22-Carbon Tricarboxylic Acid and Its Ester: A Case Study" Catalysts 9, no. 12: 972. https://doi.org/10.3390/catal9120972
APA StyleLiu, Y., Zhang, Y., Wang, L., Zan, X., & Zhang, L. (2019). The Role of Iodine Catalyst in the Synthesis of 22-Carbon Tricarboxylic Acid and Its Ester: A Case Study. Catalysts, 9(12), 972. https://doi.org/10.3390/catal9120972