Dehydration of Bioethanol to Ethylene over H-ZSM-5 Catalysts: A Scale-Up Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Lab Scale Bioethanol Dehydration
2.1.1. Effect of Si/Al Ratio of H-ZSM-5, Reaction Temperature, and Weight Hourly Space Velocity (WHSV) in Ethanol Dehydration
2.1.2. Preparation of Modified H-ZSM-5 Catalysts and Their Dehydration of Ethanol
2.2. Bench Scale Bioethanol Dehydration
2.2.1. Preparation of Molded H-ZSM-5A Catalysts and their Dehydration of Ethanol
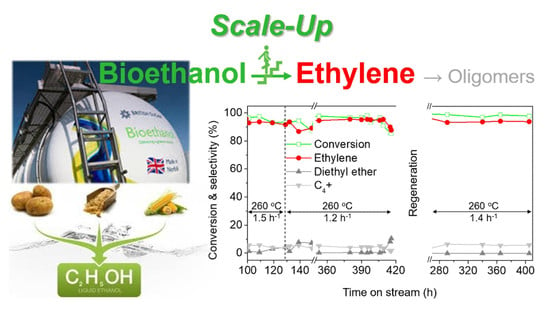
2.2.2. Long-Term Stability and Regeneration Tests of Molded H-ZSM-5A4 Catalyst
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Rye, L.; Blakey, S.; Wilson, C.W. Sustainability of supply or the planet: A review of potential drop-in alternative aviation fuels. Energy Environ. Sci. 2010, 3, 17–27. [Google Scholar] [CrossRef]
- Wang, W.-C.; Tao, L. Bio-jet fuel conversion technologies. Renew. Sust. Energ. Rev. 2016, 53, 801–822. [Google Scholar] [CrossRef]
- Byogy Renewables Inc. Available online: http://www.byogy.com/ (accessed on 28 June 2013).
- Morschbacker, A. Bio-ethanol based ethylene. J. Macromol. Sci. Polymer Rev. 2009, 49, 79–84. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, Y. Dehydration of ethanol to ethylene. Ind. Eng. Chem. Res. 2013, 52, 9505–9514. [Google Scholar] [CrossRef]
- Muraza, O. Maximizing diesel production through oligomerization: A landmark opportunity for zeolite research. Ind. Eng. Chem. Res. 2015, 54, 781–789. [Google Scholar] [CrossRef]
- Xin, H.; Li, X.; Fang, Y.; Yi, X.; Hu, W.; Chu, Y.; Zhang, F.; Zheng, A.; Zhang, H.; Li, X. Catalytic dehydration of ethanol over post-treated ZSM-5 zeolites. J. Catal. 2014, 312, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, K.; Hui, L.M.; Han, Y.-F.; Borgna, A. Structure and reactivity of phosphorous modified H-ZSM-5 catalysts for ethanol dehydration. Catal. Commum. 2009, 10, 567–571. [Google Scholar] [CrossRef]
- Ouyang, J.; Kong, F.; Su, G.; Hu, Y.; Song, Q. Catalytic conversion of bio-ethanol to ethylene over La-modified HZSM-5 catalysts in a bioreactor. Catal. Lett. 2009, 132, 64–74. [Google Scholar] [CrossRef]
- Zhan, N.; Hu, Y.; Li, H.; Yu, D.; Han, Y.; Huang, H. Lanthanum-phosphorous modified HZSM-5 catalysts in dehydration of ethanol to ethylene: a comparative analysis. Catal. Commun. 2010, 11, 633–637. [Google Scholar] [CrossRef]
- Dumrongsakda, P.; Ruangpornvisuti, V. Theoretical investigation of ethanol conversion to ethylene over H-ZSM-5 and transition metals-exchanged ZSM-5. Catal. Lett. 2012, 142, 143–149. [Google Scholar] [CrossRef]
- Bi, J.; Guo, X.; Liu, M.; Wang, X. High effective dehydration of bio-ethanol into ethylene over nanoscale HZSM-5 zeolite catalysts. Catal. Today 2010, 149, 143–147. [Google Scholar] [CrossRef]
- Li, X.; Rezaei, F.; Ludlow, D.K.; Rownaghi, A.A. Synthesis of SAPO-34@ZSM-5 and SAPO-34@silicalite-1 core-shell zeolite composites for ethanol dehydration. Ind. Eng. Chem. Res. 2018, 57, 1446–1453. [Google Scholar] [CrossRef]
- Wang, F.; Luo, M.; Xiao, W.; Cheng, X.; Long, Y. Coking behavior of a submicron MFI catalyst during ethanol dehydration to ethylene in a pilot-scale fixed-bed reactor. Appl. Catal. A Gen. 2011, 393, 161–170. [Google Scholar] [CrossRef]
- Takahara, I.; Saito, M.; Inaba, M.; Murata, K. Dehydration of ethanol into ethylene over solid acid catalysts. Catal. Lett. 2005, 105, 249–252. [Google Scholar] [CrossRef]
- Phung, T.K.; Hernández, L.P.; Lagazzo, A.; Busca, G. Dehydration of ethanol over zeolites, silica alumina and alumina: Lewis acidity, Brønsted acidity and confinement effects. Appl. Catal. A Gen. 2015, 493, 77–89. [Google Scholar] [CrossRef]
- Kondo, J.N.; Ito, K.; Yoda, E.; Wakabayashi, F.; Domen, K. An ethoxy intermediate in ethanol dehydration on Brønsted acid sites in zeolite. J. Phys. Chem. B 2005, 109, 10969–10972. [Google Scholar] [CrossRef]
- Phung, T.K.; Busca, G. Ethanol dehydration on silica-aluminas: Active sites and ethylene/diethyl ether selectivites. Catal. Commun. 2015, 68, 110–115. [Google Scholar] [CrossRef]
- Klein, T.; de Oliveira, R.; Rosset, M.; Perez-Lopez, O.W. Ethanol dehydration to diethyl ether over Cu-Fe/ZSM-5 catalysts. Catal. Commun. 2018, 104, 32–36. [Google Scholar]
- Wang, Q.L.; Giannetto, G.; Guisnet, M. Dealumination on Y zeolites with ammonium hexafluorosilicate: Effect of washing on the hydrothermal stability. Zeolites 1990, 10, 301–303. [Google Scholar] [CrossRef]
- Silaghi, M.-C.; Chizallet, C.; Raybaud, P. Challenges on molecular aspects of dealumination and desilication of zeolites. Microporous Mesoporous Mater. 2014, 191, 82–96. [Google Scholar] [CrossRef]
- Kasyanov, I.A.; Maerle, A.A.; Ivanova, I.I.; Zaikovskii, V.I. Towards understanding of the mechanism of stepwise zeolite recrystallization into micro/mesoporous materials. J. Mater. Chem. A 2014, 2, 16978–16988. [Google Scholar] [CrossRef]
- Kapustin, G.I.; Brueva, T.R.; Klyachko, A.L. Determination of the number and acid strength of acid sites in zeolites by ammonia adsorption: comparison of calorimetry and temperature-programmed desorption of ammonia. Appl. Catal. 1988, 42, 239–246. [Google Scholar] [CrossRef]
- Seo, J.-H.; Chae, H.-J.; Kim, T.-W.; Jeong, K.-E.; Kim, C.-U.; Lee, S.-B.; Jeong, S.-Y. Influence of binder on Fe-based extrudate as Fischer-Tropsch catalysts. Korean Chem. Eng. Res. 2011, 49, 726–731. [Google Scholar] [CrossRef]







| Catalyst | Si/Al 1 | BET Area (m2 g−1) 2 | Pore Volume (cm3 g−1) 4 | Relative Acid Site Density 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total | External 3 | Microporous | Total | Weak | Medium | Strong | |||
| H-ZSM-5A | 14 | 425 | 104 | 321 | - | 100 | 51 (186) | 2 (283) | 47 (383) |
| H-ZSM-5B | 12 | 384 | 141 | 243 | 0.36 | 93 | 39 (171) | 54 (253) | 0 (-) |
| H-ZSM-5C | 15 | 394 | 108 | 286 | 0.20 | 98 | 44 (187) | 5 (304) | 49 (391) |
| H-ZSM-5D | 10 | 374 | 114 | 260 | 0.27 | 92 | 42 (178) | 9 (242) | 41 (366) |
| Molded Catalyst 1 | Synthesis Composition (g) 2 | Physicochemical Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H-ZSM-5A | Inorganic Solid | Inorganic Liquid | Organic Solid (MC-40H) | BET Area (m2 g−1) 3 | Pore Volume (cm3 g−1) 5 | Compression Strength (N) 6 | ||||
| Total | External 4 | Microporous |  |  | ||||||
| H-ZSM-5A1 | 80 | Kaolinite, 10 | Ludox AS-40, 10 | 4 | 337 | 48 | 289 | 0.22 | 9 | 9 |
| H-ZSM-5A2 | 80 | Kaolinite, 10 | AS-520, 10 | 4 | 342 | 43 | 298 | 0.23 | 4 | 3 |
| H-ZSM-5A3 | 60 | Kaolinite, 10 | Ludox AS-40, 30 | 4 | 302 | 48 | 254 | 0.25 | 15 | 10 |
| H-ZSM-5A4 | 60 | Montmorillonite, 10 | Ludox AS-40, 30 | 4 | 327 | 85 | 241 | 0.29 | 30 | 24 |
| H-ZSM-5A5 | 60 | Kaofine, 10 | Ludox AS-40, 30 | 4 | 307 | 57 | 249 | 0.26 | 23 | 14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.; Chae, H.-J.; Park, M.B. Dehydration of Bioethanol to Ethylene over H-ZSM-5 Catalysts: A Scale-Up Study. Catalysts 2019, 9, 186. https://doi.org/10.3390/catal9020186
Moon S, Chae H-J, Park MB. Dehydration of Bioethanol to Ethylene over H-ZSM-5 Catalysts: A Scale-Up Study. Catalysts. 2019; 9(2):186. https://doi.org/10.3390/catal9020186
Chicago/Turabian StyleMoon, Sanggil, Ho-Jeong Chae, and Min Bum Park. 2019. "Dehydration of Bioethanol to Ethylene over H-ZSM-5 Catalysts: A Scale-Up Study" Catalysts 9, no. 2: 186. https://doi.org/10.3390/catal9020186
APA StyleMoon, S., Chae, H. -J., & Park, M. B. (2019). Dehydration of Bioethanol to Ethylene over H-ZSM-5 Catalysts: A Scale-Up Study. Catalysts, 9(2), 186. https://doi.org/10.3390/catal9020186