Self-Templating Synthesis of 3D Hierarchical NiCo2O4@NiO Nanocage from Hydrotalcites for Toluene Oxidation
Abstract
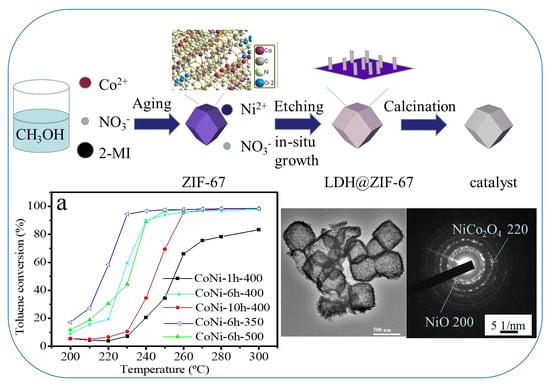
:1. Introduction
2. Results and Discussion
2.1. Textural Analysis
2.2. X-ray Photoelectron Spectra (XPS), Raman, Temperature-Programmed Reduction (TPR) and Temperature-Programmed Desorption (TPD)
2.3. Evaluation of the Catalytic Behavior
3. Materials and Methods
3.1. Raw Materials and Reagents
3.2. Synthesis of ZIF-67
3.3. Synthesis of Co-NiLDH@ZIF-67
3.4. Synthesis of NiCo2O4@NiO Nanocage
3.5. Synthesis of Single Metal Oxide Catalysts (Co and Ni)
3.6. Characterization of the Precursors and Catalysts
3.7. Catalytic Activity Measurement
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deng, J.; He, S.; Xie, S.; Yang, H.; Liu, Y.; Guo, G.; Dai, H. Ultralow Loading of Silver Nanoparticles on Mn2O3 Nanowires Derived with Molten Salts: A High-Efficiency Catalyst for the Oxidative Removal of Toluene. Environ. Sci. Technol. 2015, 49, 11089–11095. [Google Scholar] [CrossRef] [PubMed]
- Genuino, H.C.; Dharmarathna, S.; Njagi, E.C.; Mei, M.C.; Suib, S.L. Gas-Phase Total Oxidation of Benzene, Toluene, Ethylbenzene, and Xylenes Using Shape-Selective Manganese Oxide and Copper Manganese Oxide Catalysts. J. Phys. Chem. C 2012, 116, 12066–12078. [Google Scholar] [CrossRef]
- Zhang, X.; Junhui, Y.; Jing, Y.; Ting, C.; Bei, X.; Zhe, L.; Kunfeng, Z.; Ling, Y.; Dannong, H. Excellent low-temperature catalytic performance of nanosheet Co-Mn oxides for total benzene oxidation. Appl. Catal. A: Gen. 2018, 566, 104–112. [Google Scholar] [CrossRef]
- Tang, W.; Deng, Y.; Li, W.; Li, J.; Liu, G.; Li, S.; Wu, X.; Chen, Y. Importance of porous structure and synergistic effect on the catalytic oxidation activities over hierarchical Mn-Ni composite oxides. Catal. Sci. Technol. 2016, 6, 1710–1718. [Google Scholar] [CrossRef]
- Li, S.; Mo, S.; Li, J.; Liu, H.; Chen, Y. Promoted VOC oxidation over homogeneous porous CoxNiAlO composite oxides derived from hydrotalcites: Effect of preparation method and doping. Rsc Advances 2016, 6, S6874–S6884. [Google Scholar] [CrossRef]
- Zhai, G.; Wang, J.; Chen, Z.; Yang, S.; Men, Y. Highly enhanced soot oxidation activity over 3DOM Co3O4-CeO2 catalysts by synergistic promoting effect. J. Hazard. Mater. 2019, 363, 214–226. [Google Scholar] [CrossRef]
- Li, S.; Mo, S.; Wang, D.; Wu, X.; Chen, Y. Synergistic effect for promoted benzene oxidation over monolithic CoMnAlO catalysts derived from in situ supported LDH film. Catal. Today 2018. [Google Scholar] [CrossRef]
- Li, G.; Zhang, C.; Wang, Z.; Huang, H.; Peng, H.; Li, X. Fabrication of mesoporous Co3O4 oxides by acid treatment and their catalytic performances for toluene oxidation. Appl. Catal. A: Gen. 2018, 550, 67–76. [Google Scholar] [CrossRef]
- Mo, S.; Li, S.; Li, J.; Deng, Y.; Peng, S.; Chen, J.; Chen, Y. Rich surface Co(III) ions-enhanced Co nanocatalyst benzene/toluene oxidation performance derived from CoIICoIII layered double hydroxide. Nanoscale 2016, 8, 15763–15773. [Google Scholar] [CrossRef]
- Xie, R.; Fan, G.; Yang, L.; Li, F. Solvent-free oxidation of ethylbenzene over hierarchical flower-like core-shell structured Co-based mixed metal oxides with significantly enhanced catalytic performance. Catal. Sci. Technol. 2015, 5, 540–548. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Feng, J.; He, Y.; Du, Y.; Li, D. Layered double hydroxide-derived Ni-Cu nanoalloy catalysts for semihydrogenation of alkynes: Improvement of selectivity and anti-coking ability via alloying of Ni and Cu. J. Catal. 2018, 359, 251–260. [Google Scholar] [CrossRef]
- Feng, J.; He, Y.; Liu, Y.; Du, Y.; Li, D. Supported catalysts based on layered double hydroxides for catalytic oxidation and hydrogenation: General functionality and promising application prospects. Chem. Soc. Rev. 2015, 44, 5291–5319. [Google Scholar] [CrossRef]
- Mo, S.; Li, S.; Li, W.; Li, J.; Chen, J.; Chen, Y. Excellent low temperature performance for total benzene oxidation over mesoporous CoMnAl composited oxides from hydrotalcites. J. Mater. Chem. A 2016, 4, 8113–8122. [Google Scholar] [CrossRef]
- Hu, H.; Guan, B.; Xia, B.; Lou, X.W. Designed Formation of Co3O4/NiCo2O4 Double-Shelled Nanocages with Enhanced Pseudocapacitive and Electrocatalytic Properties. J. Am. Chem. Soc. 2015, 137, 5590–5595. [Google Scholar] [CrossRef]
- Sun, C.; Yang, J.; Rui, X.; Zhang, W.; Yan, Q.; Chen, P.; Huo, F.; Huang, W.; Dong, X. MOF-directed templating synthesis of a porous multicomponent dodecahedron with hollow interiors for enhanced lithium-ion battery anodes. J. Mater. Chem. A 2015, 3, 8483–8488. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, F.; Gao, L.; Duan, G. Co3O4 nanosheet-built hollow spheres containing ultrafine neck-connected grains templated by PS@Co-LDH. Sens. Actuators B 2018, 261, 553–565. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Liu, Y.; Liang, Y.; Peng, Y.; Bartlett, S.; Dai, H.; Rostamnia, S.; Li, J. Template-free Scalable Synthesis of Flower-like Co3−xMnxO4 Spinel Catalysts for Toluene Oxidation. ChemCatChem 2018, 10, 3429–3434. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y.; Xiao, L.; Yan, M.; Bai, H.; Zhu, F.; Lei, Y. Self-templated transformation of MOFs into layered double hydroxide nanoarrays. Chem. Eng. J. 2018, 354, 716–726. [Google Scholar] [CrossRef]
- Yilmaz, G.; Yam, K.M.; Zhang, C.; Fan, H.J.; Ho, G.W. In Situ Transformation of MOFs into Layered Double Hydroxide Embedded Metal Sulfides for Improved Electrocatalytic and Supercapacitive Performance. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, Z.; Qin, Z.; Sun, H.; Jiao, X.; Chen, D. LDH nanocages synthesized with MOF templates and their high performance as supercapacitors. Nanoscale 2013, 5, 11770–11775. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Botu, V.; Wang, S.; Meng, Y.; Song, W.; Guo, Y.; Ramprasad, R.; Suib, S.L.; Gao, P.X. Monolithically integrated spinel MxCo3-xO4 (M = Co, Ni, Zn) nanoarray catalysts: Scalable synthesis and cation manipulation for tunable low-temperature CH4 and CO oxidation. Angew. Chem. Int. Ed. Engl. 2014, 53, 7223–7227. [Google Scholar] [CrossRef]
- Li, R.; Che, R.; Liu, Q.; Su, S.; Li, Z.; Zhang, H.; Liu, J.; Liu, L.; Wang, J. Hierarchically structured layered-double-hydroxides derived by ZIF-67 for uranium recovery from simulated seawater. J. Hazard. Mater. 2017, 338, 167–176. [Google Scholar] [CrossRef]
- Demchenko, I.N.; Syryanyy, Y.; Melikhov, Y.; Nittler, L.; Gladczuk, L.; Lasek, K.; Cozzarini, L.; Dalmiglio, M.; Goldoni, A.; Konstantynov, P.; et al. X-ray photoelectron spectroscopy analysis as a tool to assess factors influencing magnetic anisotropy type in Co/MgO system with gold interlayer. Scripta Mater. 2018, 145, 50–53. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, J.; Yang, L.; Fan, G.; Li, F. In Situ Growth Route to Fabricate Ternary Co–Ni–Al Mixed-Metal Oxide Film as a Promising Structured Catalyst for the Oxidation of Benzyl Alcohol. Ind. Eng. Chem. Res. 2017, 56, 4237–4244. [Google Scholar] [CrossRef]
- Lv, Z.; Zhong, Q.; Bu, Y. Controllable synthesis of Ni-Co nanosheets covered hollow box via altering the concentration of nitrate for high performance supercapacitor. Electrochim. Acta 2016, 215, 500–505. [Google Scholar] [CrossRef]
- Tian, X.; Tian, C.; Nie, Y.; Dai, C.; Yang, C.; Tian, N.; Zhou, Z.; Li, Y.; Wang, Y. Controlled synthesis of dandelion-like NiCo2O4 microspheres and their catalytic performance for peroxymonosulfate activation in humic acid degradation. Chem. Eng. J. 2018, 331, 144–151. [Google Scholar] [CrossRef]
- Shao, J.; Wan, Z.; Liu, H.; Zheng, H.; Gao, T.; Shen, M.; Qu, Q.; Zheng, H. Metal organic frameworks-derived Co3O4 hollow dodecahedrons with controllable interiors as outstanding anodes for Li storage. J. Mater. Chem. A 2014, 2, 12194–12200. [Google Scholar] [CrossRef]
- Bhaway, S.M.; Tangvijitsakul, P.; Lee, J.; Soucek, M.D.; Vogt, B.D. High rate sodium ion battery anodes from block copolymer templated mesoporous nickel–cobalt carbonates and oxides. J. Mater. Chem. A 2015, 3, 21060–21069. [Google Scholar] [CrossRef]
- Su, L.; Dong, W.; Wu, C.; Gong, Y.; Zhang, Y.; Li, L.; Mao, G.; Feng, S. The peroxidase and oxidase-like activity of NiCo2O4 mesoporous spheres: Mechanistic understanding and colorimetric biosensing. Anal. Chim. Acta 2017, 951, 124–132. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.; Bai, B.; Zhao, S.; Hu, F.; Li, J. Bridging the reaction route of toluene total oxidation and the structure of ordered mesoporous Co3O4: The roles of surface sodium and adsorbed oxygen. Catal. Today 2017, 297, 173–181. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, X.; Peng, R.; Zhao, M.; Ye, D. Catalytic properties of manganese oxide polyhedra with hollow and solid morphologies in toluene removal. Appl. Surf. Sci. 2017, 405, 20–28. [Google Scholar] [CrossRef]
- Anandha Babu, G.; Ravi, G.; Hayakawa, Y. Microwave synthesis and effect of CTAB on ferromagnetic properties of NiO, Co3O4 and NiCo2O4 nanostructures. Appl. Phys. A 2014, 119, 219–232. [Google Scholar] [CrossRef]
- Liotta, L.F.; Wu, H.; Pantaleo, G.; Venezia, A.M. Co3O4 nanocrystals and Co3O4–MOx binary oxides for CO, CH4 and VOC oxidation at low temperatures: A review. Catal. Sci. Technol. 2013, 3, 3085–3102. [Google Scholar] [CrossRef]
- Cai, X.; Sun, W.; Xu, C.; Cao, L.; Yang, J. Highly selective catalytic reduction of NO via SO2/H2O-tolerant spinel catalysts at low temperature. Environ. Sci. Pollut. Res. Int. 2016, 23, 18609–18620. [Google Scholar] [CrossRef]
- Wang, B.; Cao, Y.; Chen, Y.; Wang, R.; Wang, X.; Lai, X.; Xiao, C.; Tu, J.; Ding, S. Microwave-assisted fast synthesis of hierarchical NiCo2O4 nanoflower-like supported Ni(OH)2 nanoparticles with an enhanced electrocatalytic activity towards methanol oxidation. Inorg. Chem. Frontiers 2018, 5, 172–182. [Google Scholar] [CrossRef]
- Mazzi, A.; Bazzanella, N.; Orlandi, M.; Edla, R.; Patel, N.; Fernandes, R.; Miotello, A. Physical vapor deposition of mixed-metal oxides based on Fe, Co and Ni as water oxidation catalysts. Mater. Sci. Semicond. Process. 2016, 42, 155–158. [Google Scholar] [CrossRef]
- Wang, X.; Wen, W.; Mi, J.; Li, X.; Wang, R. The ordered mesoporous transition metal oxides for selective catalytic reduction of NOx at low temperature. Appl. Catal. B: Environ. 2015, 176–177, 454–463. [Google Scholar] [CrossRef]
- Rouibah, K.; Barama, A.; Benrabaa, R.; Guerrero-Caballero, J.; Kane, T.; Vannier, R.-N.; Rubbens, A.; Löfberg, A. Dry reforming of methane on nickel-chrome, nickel-cobalt and nickel-manganese catalysts. Int. J. Hydrogen Energy 2017, 42, 29725–29734. [Google Scholar] [CrossRef]
- Bai, G.; Dai, H.; Deng, J.; Liu, Y.; Qiu, W.; Zhao, Z.; Li, X.; Yang, H. The microemulsion preparation and high catalytic performance of mesoporous NiO nanorods and nanocubes for toluene combustion. Chem. Eng. J. 2013, 219, 200–208. [Google Scholar] [CrossRef]
- Tang, C.-W.; Wang, C.-B.; Chien, S.-H. Characterization of cobalt oxides studied by FT-IR, Raman, TPR and TG-MS. Thermochim. Acta 2008, 473, 68–73. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Chen, M.; Shi, C. CoMnxOy nanosheets with molecular-scale homogeneity: An excellent catalyst for toluene combustion. Catal. Sci. Technol. 2018, 8, 459–471. [Google Scholar] [CrossRef]
- Zhou, K.; Mousavi, B.; Luo, Z.; Phatanasri, S.; Chaemchuen, S.; Verpoort, F. Characterization and properties of Zn/Co zeolitic imidazolate frameworks vs. ZIF-8 and ZIF-67. J. Mater. Chem. A 2017, 5, 952–957. [Google Scholar] [CrossRef]










| Sample | BET Surface Area (m2 g−1) | Total Pore Volume (cm3 g−1) | T90(°C) | Ni/Co | Oads/Olatt | Co3+/Co2+ | Quantity of Oxygen Species Based on O2-TPD | Acidity Based on NH3-TPD | |
|---|---|---|---|---|---|---|---|---|---|
| EDX | XPS | ||||||||
| CoNi-1h-400 | 85.5 | 0.66 | > 300 | 2.1 | 1.1 | 1.04 | 0.61 | 1.095 | 2.062 |
| CoNi-10h-400 | 126.3 | 0.95 | 259 | 2.7 | 2.1 | 0.90 | 0.67 | 1.011 | 1.730 |
| CoNi-6h-400 | 98.4 | 0.67 | 240 | 2.0 | 1.8 | 1.01 | 0.82 | 1.183 | 1.750 |
| CoNi-6h-350 | 141.6 | 0.91 | 229 | 2.5 | 1.9 | 1.32 | 1.05 | 1.185 | 1.876 |
| CoNi-6h-500 | 64.9 | 0.30 | 244 | 2.8 | 1.6 | 1,30 | 0.56 | 0.830 | 1.343 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Li, S.; Du, Y.; Wu, X.; Chen, Y. Self-Templating Synthesis of 3D Hierarchical NiCo2O4@NiO Nanocage from Hydrotalcites for Toluene Oxidation. Catalysts 2019, 9, 352. https://doi.org/10.3390/catal9040352
Wang D, Li S, Du Y, Wu X, Chen Y. Self-Templating Synthesis of 3D Hierarchical NiCo2O4@NiO Nanocage from Hydrotalcites for Toluene Oxidation. Catalysts. 2019; 9(4):352. https://doi.org/10.3390/catal9040352
Chicago/Turabian StyleWang, Dongdong, Shuangde Li, Yingchao Du, Xiaofeng Wu, and Yunfa Chen. 2019. "Self-Templating Synthesis of 3D Hierarchical NiCo2O4@NiO Nanocage from Hydrotalcites for Toluene Oxidation" Catalysts 9, no. 4: 352. https://doi.org/10.3390/catal9040352
APA StyleWang, D., Li, S., Du, Y., Wu, X., & Chen, Y. (2019). Self-Templating Synthesis of 3D Hierarchical NiCo2O4@NiO Nanocage from Hydrotalcites for Toluene Oxidation. Catalysts, 9(4), 352. https://doi.org/10.3390/catal9040352