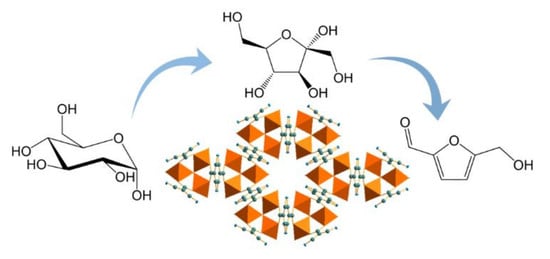
Replacement of Chromium by Non-Toxic Metals in Lewis-Acid MOFs: Assessment of Stability as Glucose Conversion Catalysts
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Synthesis of MIL-101(Fe,Sc)
4.3. Synthesis of MIL-88B(Sc)
4.4. Materials Characterisation
4.5. Catalytic Tests
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgements
Conflicts of Interest
References
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Van Putten, R.J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Teong, S.P.; Yi, G.S.; Zhang, Y.G. Hydroxymethylfurfural production from bioresources: Past, present and future. Green Chem. 2014, 16, 2015–2026. [Google Scholar] [CrossRef]
- Carson, F.; Su, J.; Platero-Prats, A.E.; Wan, W.; Yun, Y.F.; Samain, L.; Zou, X.D. Framework Isomerism in Vanadium Metal-Organic Frameworks: MIL-88B(V) and MIL-101(V). Cryst. Growth Des. 2013, 13, 5036–5044. [Google Scholar] [CrossRef]
- Roman-Leshkov, Y.; Moliner, M.; Labinger, J.A.; Davis, M.E. Mechanism of Glucose Isomerization Using a Solid Lewis Acid Catalyst in Water. Angew. Chem. Int. Edit. 2010, 49, 8954–8957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pidko, E.A.; Degirmenci, V.; van Santen, R.A.; Hensen, E.J.M. Glucose Activation by Transient Cr2+ Dimers. Angew. Chem. Int. Edit. 2010, 49, 2530–2534. [Google Scholar] [CrossRef] [PubMed]
- Pagan-Torres, Y.J.; Wang, T.F.; Gallo, J.M.R.; Shanks, B.H.; Dumesic, J.A. Production of 5-Hydroxymethylfurfural from Glucose Using a Combination of Lewis and Bronsted Acid Catalysts in Water in a Biphasic Reactor with an Alkylphenol Solvent. ACS Catal. 2012, 2, 930–934. [Google Scholar] [CrossRef]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the Interplay of Lewis and Brønsted Acid Catalysts in Glucose and Fructose Conversion to 5-(Hydroxymethyl)furfural and Levulinic Acid in Aqueous Media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef]
- Moliner, M.; Roman-Leshkov, Y.; Davis, M.E. Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. Proc. Nat. Acad. Sci. USA 2010, 107, 6164–6168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbst, A.; Janiak, C. Selective glucose conversion to 5-hydroxymethylfurfural (5-HMF) instead of levulinic acid with MIL-101Cr MOF-derivatives. New J. Chem. 2016, 40, 7958–7967. [Google Scholar] [CrossRef] [Green Version]
- Nikolla, E.; Roman-Leshkov, Y.; Moliner, M.; Davis, M.E. “One-Pot” Synthesis of 5-(Hydroxymethyl)furfural from Carbohydrates using Tin-Beta Zeolite. ACS Catal. 2011, 1, 408–410. [Google Scholar] [CrossRef] [Green Version]
- Degirmenci, V.; Hensen, E.J.M. Development of a Heterogeneous Catalyst for Lignocellulosic Biomass Conversion: Glucose Dehydration by Metal Chlorides in a Silica-Supported Ionic Liquid Layer. Environ. Prog. Sustainable Energy 2014, 33, 657–662. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.W.; Bhadra, B.N.; Ahmed, I.; Khan, N.A.; Jhung, S.H. Adsorptive Removal of Pharmaceuticals and Personal Care Products from Water with Functionalized Metal-organic Frameworks: Remarkable Adsorbents with Hydrogen-bonding Abilities. Sci. Rep. 2016, 6, 34462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Wen, H.M.; Zhou, W.; Chen, B.L. Porous Metal-Organic Frameworks for Gas Storage and Separation: What, How, and Why? J. Phys. Chem. Lett. 2014, 5, 3468–3479. [Google Scholar] [CrossRef] [PubMed]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal-Organic Frameworks: Opportunities for Catalysis. Angew. Chem. Int. Edit. 2009, 48, 7502–7513. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcia, H.; Xamena, F. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Kustov, L.M. The application of metal-organic frameworks in catalysis (Review). Petrol. Chem. 2010, 50, 167–180. [Google Scholar] [CrossRef]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal-organic frameworks: versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef]
- Herbst, A.; Janiak, C. MOF catalysts in biomass upgrading towards value-added fine chemicals. CrystEngCommun 2017, 19, 4092–4117. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional metal-organic framework catalysts: synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.C.; Yaghi, O.M. Brønsted Acidity in Metal-Organic Frameworks. Chem. Rev. 2015, 115, 6966–6997. [Google Scholar] [CrossRef]
- Goesten, M.G.; Juan-Alcaniz, J.; Ramos-Fernandez, E.V.; Gupta, K.; Stavitski, E.; van Bekkum, H.; Gascon, J.; Kapteijn, F. Sulfation of metal-organic frameworks: Opportunities for acid catalysis and proton conductivity. J. Catal. 2011, 281, 177–187. [Google Scholar] [CrossRef]
- Juan-Alcaniz, J.; Gielisse, R.; Lago, A.B.; Ramos-Fernandez, E.V.; Serra-Crespo, P.; Devic, T.; Guillou, N.; Serre, C.; Kapteijn, F.; Gascon, J. Towards acid MOFs—catalytic performance of sulfonic acid functionalized architectures. Catal. Sci. Technol. 2013, 3, 2311–2318. [Google Scholar] [CrossRef]
- Hu, Z.G.; Zhao, D. Metal-organic frameworks with Lewis acidity: Synthesis, characterization, and catalytic applications. CrystEngCommun 2017, 19, 4066–4081. [Google Scholar] [CrossRef]
- Su, Y.; Chang, G.G.; Zhang, Z.G.; Xing, H.B.; Su, B.G.; Yang, Q.W.; Ren, Q.L.; Yang, Y.W.; Bao, Z.B. Catalytic dehydration of glucose to 5-hydroxymethylfurfural with a bifunctional metal-organic framework. AICHE J. 2016, 62, 4403–4417. [Google Scholar] [CrossRef]
- Chen, J.Z.; Li, K.G.; Chen, L.M.; Liu, R.L.; Huang, X.; Ye, D.Q. Conversion of fructose into 5-hydroxymethylfurfural catalyzed by recyclable sulfonic acid-functionalized metal-organic frameworks. Green Chem. 2014, 16, 2490–2499. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Degirmenci, V.; Li, C.; Hensen, E.J.M. Phosphotungstic Acid Encapsulated in Metal-Organic Framework as Catalysts for Carbohydrate Dehydration to 5-Hydroxymethylfurfural. ChemSusChem 2011, 4, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Ren, L.M.; Kumar, P.; Cybulskis, V.J.; Mkhoyan, K.A.; Davis, M.E.; Tsapatsis, M. A Chromium Hydroxide/MIL-101(Cr) MOF Composite Catalyst and Its Use for the Selective Isomerization of Glucose to Fructose. Angew. Chem. Int. Edit. 2018, 57, 4926–4930. [Google Scholar] [CrossRef] [PubMed]
- Yabushita, M.; Li, P.; Islamoglu, T.; Kobayashi, H.; Fukuoka, A.; Farha, O.K.; Katz, A. Selective Metal-Organic Framework Catalysis of Glucose to 5-Hydroxymethylfurfural Using Phosphate-Modified NU-1000. Ind. Eng. Chem. Res. 2017, 56, 7141–7148. [Google Scholar] [CrossRef]
- Oozeerally, R.; Burnett, D.L.; Chamberlain, T.W.; Walton, R.I.; Degirmenci, V. Exceptionally Efficient and Recylable Heterogeneous Metal-Organic Framework Catalyst for Glucose Isomerisation in Water. ChemCatChem 2018, 10, 706–709. [Google Scholar] [CrossRef]
- De Mello, M.D.; Tsapatsis, M. Selective Glucose-to-Fructose Isomerization over Modified Zirconium UiO-66 in Alcohol Media. ChemCatChem 2018, 10, 2417–2423. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Jin, P.; Meng, M.J.; Gao, L.; Liu, M.; Yan, Y.S. Acid-Base Bifunctional Metal-Organic Frameworks: Green Synthesis and Application in One-Pot Glucose to 5-HMF Conversion. Nano 2018, 13. [Google Scholar] [CrossRef]
- Gong, J.; Katz, M.J.; Kerton, F.M. Catalytic conversion of glucose to 5-hydroxymethylfurfural using zirconium-containing metal-organic frameworks using microwave heating. RSC Adv. 2018, 8, 31618–31627. [Google Scholar] [CrossRef]
- Desidery, L.; Yusubov, M.S.; Zhuiykov, S.; Verpoort, F. Fully-sulfonated hydrated UiO-66 as efficient catalyst for ethyl levulinate production by esterification. Catal. Commun. 2018, 117, 33–37. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Dayan, A.D.; Paine, A.J. Mechanisms of chromium toxicity, carcinogenicity and allergenicity: Review of the literature from 1985 to 2000. Hum. Exp. Toxicol. 2001, 20, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Kubota, M.; Kurihara, H.; Yamamoto, H. Scandium trifluoromethanesulfonate as an extremely active Lewis acid catalyst in acylation of alcohols with acid anhydrides and mixed anhydrides. J. Org. Chem. 1996, 61, 4560–4567. [Google Scholar] [CrossRef]
- Mowat, J.P.S.; Miller, S.R.; Slawin, A.M.Z.; Seymour, V.R.; Ashbrook, S.E.; Wright, P.A. Synthesis, characterisation and adsorption properties of microporous scandium carboxylates with rigid and flexible frameworks. Micropor. Mesopor. Mater. 2011, 142, 322–333. [Google Scholar] [CrossRef]
- Mitchell, L.; Gonzalez-Santiago, B.; Mowat, J.P.S.; Gunn, M.E.; Williamson, P.; Acerbi, N.; Clarke, M.L.; Wright, P.A. Remarkable Lewis acid catalytic performance of the scandium trimesate metal organic framework MIL-100(Sc) for C-C and C=N bond-forming reactions. Catal. Sci. Technol. 2013, 3, 606–617. [Google Scholar] [CrossRef]
- Mitchell, L.; Williamson, P.; Ehrlichova, B.; Anderson, A.E.; Seymour, V.R.; Ashbrook, S.E.; Acerbi, N.; Daniels, L.M.; Walton, R.I.; Clarke, M.L.; Wright, P.A. Mixed-Metal MIL-100(Sc,M) (M=Al, Cr, Fe) for Lewis Acid Catalysis and Tandem C-C Bond Formation and Alcohol Oxidation. Chem. Eur. J. 2014, 20, 17185–17197. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, T.; Boldog, I.; Janiak, C.; Yang, X.Y.; Li, Q.; Zhou, Y.J.; Xia, Y.; Lai, D.W.; Liu, Y.J. Benzoic acid as a selector-modulator in the synthesis of MIL-88B(Cr) and nano-MIL-101(Cr). Dalton Trans. 2019, 48, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.T.; Zhang, Z.M.; Chen, W.L.; Liu, Z.J.; Wang, E.B. Encapsulation of tungstophosphoric acid into harmless MIL-101(Fe) for effectively removing cationic dye from aqueous solution. RSC Adv. 2016, 6, 81622–81630. [Google Scholar] [CrossRef]
- Bromberg, L.; Diao, Y.; Wu, H.M.; Speakman, S.A.; Hatton, T.A. Chromium(III) Terephthalate Metal Organic Framework (MIL-101): HF-Free Synthesis, Structure, Polyoxometalate Composites, and Catalytic Properties. Chem. Mater. 2012, 24, 1664–1675. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Zhang, C.L.; Yang, Z.; Dai, X.P.; Cheng, M.S.; Hou, X.H. Metal-organic framework MIL-101(Cr) as a sorbent of porous membrane-protected micro-solid-phase extraction for the analysis of six phthalate esters from drinking water: a combination of experimental and computational study. Analyst 2015, 140, 5308–5316. [Google Scholar] [CrossRef] [PubMed]
- Montazerolghaem, M.; Aghamiri, S.F.; Tangestaninejad, S.; Talaie, M.R. A metal-organic framework MIL-101 doped with metal nanoparticles (Ni & Cu) and its effect on CO2 adsorption properties. RSC Adv. 2016, 6, 632–640. [Google Scholar]
- Surblé, S.; Serre, C.; Mellot-Draznieks, C.; Millange, F.; Férey, G. A new isoreticular class of metal-organic-frameworks with the MIL-88 topology. Chem. Commun. 2006, 284–286. [Google Scholar] [CrossRef]
- Bauer, S.; Serre, C.; Devic, T.; Horcajada, P.; Marrot, J.; Férey, G.; Stock, N. High-throughput assisted rationalization of the formation of metal organic frameworks in the iron(III) aminoterephthalate solvothermal system. Inorg. Chem. 2008, 47, 7568–7576. [Google Scholar] [CrossRef]
- Tanasaro, T.; Adpakpang, K.; Ittisanronnachai, S.; Faungnawakij, K.; Butburee, T.; Wannapaiboon, S.; Ogawa, M.; Bureekaew, S. Control of Polymorphism of Metal-Organic Frameworks Using Mixed-Metal Approach. Cryst. Growth Des. 2018, 18, 16–21. [Google Scholar] [CrossRef]
- Ma, M.Y.; Betard, A.; Weber, I.; Al-Hokbany, N.S.; Fischer, R.A.; Metzler-Nolte, N. Iron-Based Metal-Organic Frameworks MIL-88B and NH2-MIL-88B: High Quality Microwave Synthesis and Solvent-Induced Lattice “Breathing”. Cryst. Growth Des. 2013, 13, 2286–2291. [Google Scholar] [CrossRef]
- Serre, C.; Mellot-Draznieks, C.; Surblé, S.; Audebrand, N.; Filinchuk, Y.; Férey, G. Role of solvent-host interactions that lead to very large swelling of hybrid frameworks. Science 2007, 315, 1828–1831. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.P.; Sen, S.K. Calculation of multiplet structure of core p -vacancy levels. II. Phys. Rev. B 1975, 12, 15–19. [Google Scholar] [CrossRef]
- Gota, S.; Guiot, E.; Henriot, M.; Gautier-Soyer, M. Atomic-oxygen-assisted MBE growth of a-Fe2O3 on a-Al2O3 (0001): Metastable FeO(111)-like phase at subnanometer thicknesses. Phys. Rev. B 1999, 60, 14387–14395. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Jimenez-Cavero, P.; Lucas, I.; Anadon, A.; Ramos, R.; Niizeki, T.; Aguirre, M.H.; Algarabel, P.A.; Uchida, K.; Ibarra, M.R.; Saitoh, E.; Morellon, L. Spin Seebeck effect in insulating epitaxial gamma-Fe2O3 thin films. APL Mater. 2017, 5. [Google Scholar]
- Jia, S.Y.; Xu, Z.W.; Zhang, Z.C. Catalytic conversion of glucose in dimethylsulfoxide/water binary mix with chromium trichloride: Role of water on the product distribution. Chem. Eng. J. 2014, 254, 333–339. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Williams, L.D.; Ebede, C.C. Mechanism of the dehydration of D-fructose to 5-hydroxymethylfurfural in dimethyl sulfoxide at 150 °C: An NMR study. Carbohydr. Res. 2008, 343, 3021–3024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swift, T.D.; Nguyen, H.; Anderko, A.; Nikolakis, V.; Vlachos, D.G. Tandem Lewis/Brønsted homogeneous acid catalysis: Conversion of glucose to 5-hydoxymethylfurfural in an aqueous chromium(III) chloride and hydrochloric acid solution. Green Chem. 2015, 17, 4725–4735. [Google Scholar] [CrossRef]
- Li, K.; Du, M.M.; Ji, P.J. Multifunctional Tin-Based Heterogeneous Catalyst for Catalytic Conversion of Glucose to 5-Hydroxymethylfurfural. ACS Sust. Chem. Eng. 2018, 6, 5636–5644. [Google Scholar] [CrossRef]
- Behera, G.C.; Parida, K.M. One-pot synthesis of 5-hydroxymethylfurfural: a significant biomass conversion over tin-promoted vanadium phosphate (Sn-VPO) catalyst. Catal. Sci. Technol. 2013, 3, 3278–3285. [Google Scholar] [CrossRef]
- Ngee, E.L.S.; Gao, Y.J.; Chen, X.; Lee, T.M.; Hu, Z.G.; Zhao, D.; Yan, N. Sulfated Mesoporous Niobium Oxide Catalyzed 5-Hydroxymethylfurfural Formation from Sugars. Ind. Eng. Chem. Res. 2014, 53, 14225–14233. [Google Scholar] [CrossRef]
- Yan, H.P.; Yang, Y.; Tong, D.M.; Xiang, X.; Hu, C.W. Catalytic conversion of glucose to 5-hydroxymethylfurfural over SO42-/ZrO2 and SO42-/ZrO2-Al2O3 solid acid catalysts. Catal. Commun. 2009, 10, 1558–1563. [Google Scholar] [CrossRef]
- Jiang, H.X.; Zhou, J.L.; Wang, C.X.; Li, Y.H.; Chen, Y.F.; Zhang, M.H. Effect of Cosolvent and Temperature on the Structures and Properties of Cu-MOF-74 in Low-temperature NH3-SCR. Ind. Eng. Chem. Res. 2017, 56, 3542–3550. [Google Scholar] [CrossRef]
- Rahmani, E.; Rahmani, M. Alkylation of benzene over Fe-based metal organic frameworks (MOFs) at low temperature condition. Micropor. Mesopor. Mater. 2017, 249, 118–127. [Google Scholar] [CrossRef]
- Vuong, G.T.; Pham, M.H.; Do, T.O. Synthesis and engineering porosity of a mixed metal Fe2Ni MIL-88B metal-organic framework. Dalton Trans. 2013, 42, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Perles, J.; Iglesias, M.; Ruiz-Valero, C.; Snejko, N. Rare-earths as catalytic centres in organo-inorganic polymeric frameworks. J. Mater. Chem. 2004, 14, 2683–2689. [Google Scholar] [CrossRef]
- Gandara, F.; Gomez-Lor, B.; Iglesias, M.; Snejko, N.; Gutierrez-Puebla, E.; Monge, A. A new scandium metal organic framework built up from octadecasil zeolitic cages as heterogeneous catalyst. Chem. Commun. 2009, 2393–2395. [Google Scholar] [CrossRef]
- Larson, A.C.; Dreele, R.B.V. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report 1994, LAUR 86–748; Los Alamos National Laboratory: Los Alamos, NM, USA, 1994. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Cryst. 2001, 34, 210–213. [Google Scholar] [CrossRef]










| Temperature (°C) | Substrate to Catalyst Ratio (wt./wt.) | Catalyst | Conversion (%) | Product Yield (% Mole) | Product Selectivity (% Mole) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fructose | Mannose | 5-HMF | Fructose | Mannose | 5-HMF | ||||
| 120 | none | 1.6 | 0.0 | 0.0 | 0.01 | 0.0 | 0.0 | 0.69 | |
| 30:1 | MIL-88B(Fe) | 18.9 | 6.3 | 0.3 | 0.0 | 33.3 | 1.6 | 0.0 | |
| MIL-88B(Sc) | 9.1 | 1.0 | 0.4 | 0.1 | 11.0 | 4.4 | 1.1 | ||
| MIL-88B(Fe,Sc) | 11.5 | 1.1 | 0.2 | 0.7 | 9.6 | 1.7 | 6.1 | ||
| 140 | none | 60.3 | 0.0 | 0.0 | 0.6 | 0.0 | 0.0 | 1.0 | |
| 30:1 | MIL-88B(Fe) | 54.2 | 7.6 | 1.2 | 0.9 | 14.0 | 2.2 | 1.7 | |
| MIL-88B(Sc) | 28.8 | 2.1 | 1.1 | 3.2 | 7.3 | 3.8 | 11.1 | ||
| MIL-88B(Fe,Sc) | 53.3 | 3.6 | 2.0 | 17.8 | 6.8 | 3.8 | 33.4 | ||
| 120 | 7.5:1 | MIL-88B(Fe) | 24.2 | 3.2 | 0.0 | 0.4 | 13.2 | 0.0 | 1.7 |
| MIL-88B(Fe,Sc) | 19.7 | 0.8 | 2.3 | 3.3 | 4.1 | 11.7 | 16.8 | ||
| 140 | 7.5:1 | MIL-88B(Fe) | 55.6 | 3.6 | 1.0 | 3.8 | 6.5 | 1.8 | 6.8 |
| MIL-88B(Fe,Sc) | 70.7 | 3.3 | 1.4 | 24.9 | 4.7 | 2.0 | 35.2 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pertiwi, R.; Oozeerally, R.; Burnett, D.L.; Chamberlain, T.W.; Cherkasov, N.; Walker, M.; Kashtiban, R.J.; Krisnandi, Y.K.; Degirmenci, V.; Walton, R.I. Replacement of Chromium by Non-Toxic Metals in Lewis-Acid MOFs: Assessment of Stability as Glucose Conversion Catalysts. Catalysts 2019, 9, 437. https://doi.org/10.3390/catal9050437
Pertiwi R, Oozeerally R, Burnett DL, Chamberlain TW, Cherkasov N, Walker M, Kashtiban RJ, Krisnandi YK, Degirmenci V, Walton RI. Replacement of Chromium by Non-Toxic Metals in Lewis-Acid MOFs: Assessment of Stability as Glucose Conversion Catalysts. Catalysts. 2019; 9(5):437. https://doi.org/10.3390/catal9050437
Chicago/Turabian StylePertiwi, Ralentri, Ryan Oozeerally, David L. Burnett, Thomas W. Chamberlain, Nikolay Cherkasov, Marc Walker, Reza J. Kashtiban, Yuni K. Krisnandi, Volkan Degirmenci, and Richard I. Walton. 2019. "Replacement of Chromium by Non-Toxic Metals in Lewis-Acid MOFs: Assessment of Stability as Glucose Conversion Catalysts" Catalysts 9, no. 5: 437. https://doi.org/10.3390/catal9050437
APA StylePertiwi, R., Oozeerally, R., Burnett, D. L., Chamberlain, T. W., Cherkasov, N., Walker, M., Kashtiban, R. J., Krisnandi, Y. K., Degirmenci, V., & Walton, R. I. (2019). Replacement of Chromium by Non-Toxic Metals in Lewis-Acid MOFs: Assessment of Stability as Glucose Conversion Catalysts. Catalysts, 9(5), 437. https://doi.org/10.3390/catal9050437