Facile Mechanochemical Synthesis of Nickel/Graphene Oxide Nanocomposites with Unique and Tunable Morphology: Applications in Heterogeneous Catalysis and Supercapacitors
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Ni/GO Nanocomposites
2.3. Characterization
2.4. Procedure for the Reduction of Nitrophenols
2.5. Electrochemical Studies
3. Results and Discussion
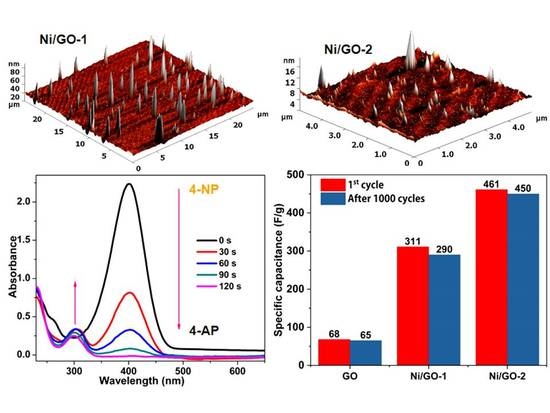
3.1. Characterization of Ni/GO Nanocomposites
3.2. Catalytic Conversion of 4- and 2-Nitrophenol
3.3. Electrochemical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rao, C.E.E.; Sood, A.E.; Subrahmanyam, K.E.; Govindaraj, A. Graphene: The new two-dimensional nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, Q.; Shi, G. Graphene based new energy materials. Energy Environ. Sci. 2011, 4, 1113. [Google Scholar] [CrossRef]
- Qin, Z.; Taylor, M.; Hwang, M.; Bertoldi, K.; Buehler, M. Effect of Wrinkles on the Surface Area of Graphene: Toward the Design of Nanoelectronics. Nano Lett. 2014, 14, 6520–6525. [Google Scholar] [CrossRef] [Green Version]
- Frank, O.; Tsoukleri, G.; Riaz, I.; Papagelis, K.; Parthenios, J.; Ferrari, A.C.; Geim, A.K.; Novoselov, K.S.; Galiotis, C. Development of a universal stress sensor for graphene and carbon fibres. Nat. Commun. 2011, 2, 255. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.J.; Balakrishnan, K.; Huang, J.; Meunier, V.; Sumpter, B.G.; Srivastava, A.; Conway, M.; Mohana Reddy, A.L.; Yu, J.; Vajtai, R.; et al. Ultrathin planar graphenesupercapacitors. Nano Lett. 2011, 11, 1423–1427. [Google Scholar] [CrossRef]
- Luo, J.; Jang, H.D.; Huang, J. Effect of sheet morphology on the scalability of graphene-based ultracapacitors. ACS Nano 2013, 7, 1464–1471. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Coelho, B.; Oliveira, A.C.; Mendes, A.; Oliveira, A. Concentrated solar power for renewable electricity and hydrogen production from water-a review. Energy Environ. Sci. 2010, 3, 1398. [Google Scholar] [CrossRef]
- Feng, X.; Liu, M.; Pisula, W.; Takase, M.; Li, J.; Müllen, K. Supramolecular organization and photovoltaics of triangle-shaped discoticgraphenes with swallow-tailed alkyl substituents. Adv. Mater. 2008, 20, 2684–2689. [Google Scholar] [CrossRef] [PubMed]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Carbocatalysis by Graphene-Based Materials. Chem. Rev. 2014, 114, 6179–6212. [Google Scholar] [CrossRef]
- Yan, H.; Cheng, H.; Yi, H.; Lin, Y.; Yao, T.; Wang, C.; Li, J.; Wei, S.; Lu, J. Single-Atom Pd 1 /Graphene Catalyst Achieved by Atomic Layer Deposition: Remarkable Performance in Selective Hydrogenation of 1,3-Butadiene. J. Am. Chem. Soc. 2015, 137, 10484–10487. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.K.; Shin, D.; Ryoo, S.R.; Hong, B.H.; Min, D.H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Pang, F.; Wang, F.; Zhang, X. Green synthesis of nanostructed Ni-reduced graphene oxide hybrids and their application for catalytic reduction of 4-nitrophenol. Colloids Surf. A 2015, 464, 96–103. [Google Scholar] [CrossRef]
- Pahalagedara, M.N.; Pahalagedara, L.R.; He, J.; Miao, R.; Gottlieb, B.; Rathnayake, D.; Suib, S.L. Room temperature selective reduction of nitrobenzene to azoxybenzene over magnetically separable urchin-like Ni/Graphene nanocomposites. J. Catal. 2016, 336, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Salimian, M.; Deepak, F.L.; Petrovykh, D.Y.; Ferro, M.; Titus, E.; Ivanov, M.; Bdikin, I.; Kholkin, A.; Goncalves, G. Synthesis and characterization of reduced graphene oxide/spiky nickel nanocomposite for nanoelectronic applications. J. Mater. Chem. C 2015, 3, 11516–11523. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, J.; Yang, Q.; Tong, L.; Zhang, J.; Zhang, J.; Gong, C.; Zhou, J.; Zhang, Z. Preparation of magnetic Ni@graphenenanocomposites and efficient removal organic dye under assistance of ultrasound. Appl. Surf. Sci. 2015, 357, 22–30. [Google Scholar] [CrossRef]
- Zhou, C.; Szpunar, J.A.; Cui, X. Synthesis of Ni/Graphenenanocomposite for hydrogen storage. ACS Appl. Mater. Interfaces 2016, 8, 15232–15241. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.C.; Chen, D.H. Ni/reduced graphene oxide nanocomposite as a magnetically recoverable catalyst with near infrared photothermally enhanced activity. Appl. Catal. B 2014, 150, 298–304. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, Y.; Shen, X.; Ma, H.; Yang, J.; Yuan, A.; Zhou, H. Facile synthesis and enhanced catalytic performance of reduced graphene oxide decorated with hexagonal structure Ni nanoparticles. J. Colloid Interface Sci. 2017, 487, 223–230. [Google Scholar] [CrossRef]
- Chen, T.; Deng, F.; Zhu, J.; Chen, C.; Sun, G.; Ma, S.; Yang, X. Hexagonal and cubic Ni nanocrystals grown on graphene: phase-controlled synthesis, characterization and their enhanced microwave absorption properties. J. Mater. Chem. 2012, 22, 15190–15197. [Google Scholar] [CrossRef]
- Kollu, P.; Prathapani, S.; Varaprasadarao, E.K.; Santosh, C.; Mallick, S.; Grace, A.N.; Bahadur, D. Anomalous magnetic behavior in nanocomposite materials of reduced graphene oxide-Ni/NiFe2O4. Appl. Phys. Lett. 2014, 105, 52412. [Google Scholar] [CrossRef]
- Ji, Z.; Shen, X.; Zhu, G.; Zhou, H.; Yuan, A. Reduced graphene oxide/nickel nanocomposites: facile synthesis, magnetic and catalytic properties. J. Mater. Chem. 2012, 22, 3471. [Google Scholar] [CrossRef]
- Li, B.; Cao, H.; Yin, J.; Wu, Y.A.; Warner, J.H. Synthesis and separation of dyes via Ni@reducedgraphene oxide nanostructures. J. Mater. Chem. 2012, 22, 1876–1883. [Google Scholar] [CrossRef]
- Ren, Z.; Meng, N.; Shehzad, K.; Xu, Y.; Qu, S.; Yu, B.; Luo, J.K. Mechanical properties of nickel-graphene composites synthesized by electrochemical deposition. Nanotechnology 2015, 26, 65706. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Su, Q.; Che, R.; Du, G.; Xu, B. One-step chemical vapor synthesis of Ni/graphenenanocomposites with excellent electromagnetic and electrocatalytic properties. Synth. Met. 2012, 162, 968–973. [Google Scholar] [CrossRef]
- Yang, F.; Deng, D.; Pan, X.; Fu, Q.; Bao, X. Understanding nano effects in catalysis. Natl. Sci. Rev. 2015, 2, 183–201. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Zhang, L.; Wang, Y.; Shi, Z.; Shi, D.; Gao, H.; Wang, E.; Zhang, G. An Anisotropic Etching Effect in the Graphene Basal Plane. Adv. Mater. 2010, 22, 4014–4019. [Google Scholar] [CrossRef]
- Zaid, N.A.M.; Idris, N.H. Enhanced Capacitance of Hybrid Layered Graphene/Nickel Nanocomposite for Supercapacitors. Sci. Rep. 2016, 6, 32082. [Google Scholar] [CrossRef]
- Gopiraman, M.; Babu, S.G.; Khatri, Z.; Kai, W.; Kim, Y.A.; Endo, M.; Karvembu, R.; Kim, I.S. Dry Synthesis of Easily Tunable Nano Ruthenium Supported on Graphene: Novel Nanocatalysts for Aerial Oxidation of Alcohols and Transfer Hydrogenation of Ketones. J. Phys. Chem. C 2013, 117, 23582–23596. [Google Scholar] [CrossRef]
- Gopiraman, M.; Babu, S.G.; Khatri, Z.; Kai, W.; Kim, Y.A.; Endo, M.; Karvembu, R.; Kim, I.S. An efficient, reusable copper-oxide/carbon-nanotube catalyst for N-arylation of imidazole. Carbon 2013, 62, 135–148. [Google Scholar] [CrossRef]
- Gopiraman, M.; Karvembu, R.; Kim, I.S. Highly Active, Selective, and Reusable RuO2/SWCNT Catalyst for Heck Olefination of Aryl Halides. ACS Catal. 2014, 4, 2118–2129. [Google Scholar] [CrossRef]
- Joo, S.H.; Park, J.Y.; Renzas, J.R.; Butcher, D.R.; Huang, W.; Somorjai, G.A. Size Effect of Ruthenium Nanoparticles in Catalytic Carbon Monoxide Oxidation. Nano Lett. 2010, 10, 2709–2713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, J.; Wang, D.; Dou, S.; Ma, Z.; Wu, J.; Tao, L.; Shen, A.; Ouyang, C.; Liu, Q.; et al. One-pot synthesis of nitrogen and sulfur co-doped graphene as efficient metal-free electrocatalysts for the oxygen reduction reaction. Chem. Commun. 2014, 50, 4839–4842. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Y.L.T.; Hur, S.H. Low-temperature NO2 gas sensor fabricated with NiO and reducedgraphene oxide hybrid structure. Mater. Res. Bull. 2016, 84, 168–176. [Google Scholar] [CrossRef]
- Gopiraman, M.; Chung, I.M. Highly active and cost-effective CuO-based carbon nanocomposite with unique morphology for catalytic synthesis of imines under solvent-free conditions. J. Taiwan. Inst. Chem. E 2017, 81, 455–464. [Google Scholar] [CrossRef]
- Sun, X.; Lu, H.; Liu, P.; Rufford, T.E.; Gaddam, R.R.; Fan, X.; Zhao, X.S. A reduced graphene oxide–NiO composite electrode with a high and stable capacitance. Sustain. Energ. Fuels 2018, 2, 673–678. [Google Scholar]
- Huang, W.; Ding, S.; Chen, Y.; Hao, W.; Lai, X.; Peng, J.; Tu, J.; Cao, Y.; Li, X. 3D NiO hollow sphere/reduced graphene oxide composite for high-performance glucose biosensor. Sci. Rep. 2017, 7, 5220. [Google Scholar] [CrossRef] [PubMed]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Heine, C.; Lechner, B.A.J.; Bluhm, H.; Salmeron, M. Recycling of CO2: Probing the Chemical State of the Ni(111) Surface during the Methanation Reaction with Ambient-Pressure X-Ray Photoelectron Spectroscopy. J. Am. Chem. Soc. 2016, 138, 13246–13252. [Google Scholar] [CrossRef]
- Somasundaram, S.; Ill-Min, C.; Vanaraj, R.; Ramaganthan, B.; Mayakrishnan, G. Highly active and reducing agent-free preparation of cost-effective NiO-based carbon nanocomposite and its application in reduction reactions under mild conditions. J. Ind. Eng. Chem. 2018, 60, 91–101. [Google Scholar] [CrossRef]
- Hudson, M.S.L.; Raghubanshi, H.; Awasthi, S.; Sadhasivam, T.; Bhatnager, A.; Simizu, S.; Sankar, S.; Srivastava, O. Hydrogen uptake of reduced graphene oxide and graphene sheets decorated with Fe nanoclusters. Int. J. Hydrog. Energy 2014, 39, 8311–8320. [Google Scholar] [CrossRef]
- Gopiraman, M.; Deng, D.; Saravanamoorthy, S.; Chung, I.-M.; Kim, I.S. Gold, silver and nickel nanoparticle anchored cellulose nanofiber composites as highly active catalysts for the rapid and selective reduction of nitrophenols in water. RSC Adv. 2018, 8, 3014–3023. [Google Scholar] [CrossRef] [Green Version]
- Gopiraman, M.; Muneeswaran, M.; Kim, I.S. Highly porous Ru/C and Cu/C nanocatalysts derived from custard apple for rapid and selective reduction of p-nitrophenol. Nano Prog. 2019, 1, 30–36. [Google Scholar]
- Ji, T.; Chen, L.; Schmitz, M.; Bao, F.S.; Zhu, J. Hierarchical macrotube/mesopore carbon decorated with mono-dispersed Ag nanoparticles as a highly active catalyst. Green Chem. 2015, 17, 2515–2523. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Astruc, Basic concepts and recent advances in nitrophenol reduction by gold- and other transition metal nanoparticles. Coordin. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Panigrahi, S.; Basu, S.; Praharaj, S.; Pande, S.; Jana, S.; Pal, A.; Ghosh, S.K.; Pal, T. Synthesis and Size-Selective Catalysis by Supported Gold Nanoparticles: Study on Heterogeneous and Homogeneous Catalytic Process. J. Phys. Chem. C 2007, 111, 4596–4605. [Google Scholar] [CrossRef]
- Kong, W.; Hu, Z.; Zhao, F.; Liu, J.; Zhang, B. Tuning the performance of Pt–Ni alloy/reduced graphene oxide catalysts for 4-nitrophenol reduction. RSC Adv. 2016, 6, 79028–79036. [Google Scholar]
- Hareesh, K.; Joshi, R.P.; Sunitha, D.V.; Bhoraskar, V.N.; Dhole, S.D. Anchoring of Ag-Au alloy nanoparticles on reduced graphene oxide sheets for the reduction of 4-nitrophenol. Appl. Surf. Sci. 2016, 389, 1050–1055. [Google Scholar]
- Zhang, Y.; Yuan, X.; Wang, Y.; Chen, Y. One-pot photochemical synthesis of graphene composites uniformly deposited with silver nanoparticles and their high catalytic activity towards the reduction of 2-nitroaniline. J. Mater. Chem. 2012, 22, 7245. [Google Scholar] [CrossRef]
- Vellaichamy, B.; Periakaruppan, P.; Thomas, J. Synthesis of AuNPs@RGOnanosheets for sustainable catalysis toward nitrophenols reduction. Ultrason. Sonochem. 2018, 48, 362–369. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, H.; Liu, Y.; Ren, Z.; Lin, C.; Tao, J.; Zhai, Y. Facile synthesis of PdNiP/Reduced graphene oxide nanocomposites for catalytic reduction of 4-nitrophenol. Mater. Chem. Phys. 2019, 222, 91–397. [Google Scholar] [CrossRef]
- Jiang, Z.; Xie, J.; Jiang, D.; Jing, J.; Qin, H. Facile route fabrication of nano-Ni core mesoporous-silica shell particles with high catalytic activity towards 4-nitrophenol reduction. Cryst. Eng. Comm. 2012, 14, 4601–4611. [Google Scholar] [CrossRef]
- Mohamed, M.J.S.; Denthaje, K.B. Novel RGO-ZnWO4-Fe3O4Nanocomposite as an Efficient Catalyst for Rapid Reduction of 4-Nitrophenol to 4-Aminophenol. Ind. Eng. Chem. Res. 2016, 55, 7267–7272. [Google Scholar] [CrossRef]
- Zhang, S.; Gai, S.; He, F.; Ding, S.; Li, L.; Yang, P. In situ assembly of well-dispersed Ni nanoparticles on silica nanotubes and excellent catalytic activity in 4-nitrophenol reduction. Nanoscale 2014, 6, 11181–11188. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, Y.; Sun, C.; Hao, S. Facile route fabrication of nickel based mesoporous carbons with high catalytic performance towards 4-nitrophenol reduction. Green Chem. 2014, 16, 2273–2280. [Google Scholar] [CrossRef]
- Gopiraman, M.; Chung, I.-M. Multifunctional human-hair nanocomposites for oxidation of alcohols, aza-Michael reactions and reduction of 2-nitrophenol. Korean J. Chem. Eng. 2017, 34, 2169–2179. [Google Scholar] [CrossRef]
- Kuroda, K.; Ishida, T.; Haruta, M. Reduction of 4-nitrophenol to 4-aminophenol over Au nanoparticles deposited on PMMA. J. Mol. Catal. A Chem. 2009, 298, 7–11. [Google Scholar] [CrossRef]
- Chen, H.; Cong, T.N.; Yang, W.; Tan, C.; Li, Y.; Ding, Y. Progress in electrical energy storage system: A critical review. Prog. Nat. Sci. Mater. 2009, 19, 291–312. [Google Scholar] [CrossRef]
- Kuperman, A.; Aharon, I. Battery–ultracapacitor hybrids for pulsed current loads: A review. Renew. Sustain. Energy Rev. 2011, 15, 981–992. [Google Scholar] [CrossRef]
- Kim, S.-I.; Lee, J.-S.; Ahn, H.-J.; Song, H.-K.; Jang, J.-H. Facile Route to an Efficient NiOSupercapacitor with a Three-Dimensional Nanonetwork Morphology. ACS Appl. Mater. Interfaces 2013, 5, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Deng, S.; Wang, H.; Sun, Y.; Liu, J.; Yan, H. Preparation of Novel Three-Dimensional NiO/Ultrathin Derived Graphene Hybrid for Supercapacitor Applications. ACS Appl. Mater. Interfaces 2014, 6, 1106–1112. [Google Scholar] [CrossRef] [PubMed]













| S. No | Catalyst (amount used, mg) | Reactant | kapp (× 10−3 s−1) | k′ (× 10−3 mg−1 s−1) | TOF a (s−1) | References |
|---|---|---|---|---|---|---|
| 1 | 23 wt.% Ni/SNTs | 4-NP | 84.0 | 91.0 | - | [55] |
| 2 | 15 wt.% Ni/SNTs | 4-NP | 20.0 | 44.0 | - | [55] |
| 3 | Ni/GO-1 (0.75) | 4-NP | 28.1 | 14.0 | 31.66 | This work |
| 4 | Ni/GO-2 (0.75) | 4-NP | 35.4 | 47.2 | 25.33 | This work |
| 5 | Ni/GO-1 (0.75) | 2-NP | 15.5 | 7.5 | 18.09 | This work |
| 6 | Ni/GO-2 (0.75) | 2-NP | 70.7 | 94.3 | 42.22 | This work |
| 7 | Ni/MC-750 (3) | 4-NP | 6.26 | 20.9 | 1.44 | [56] |
| 8 | RGO/Ni (6.5) | 4-NP | 0.25 | 0.04 | - | [57] |
| 9 | Pt–Ni/RGO (3) | 4-NP | 3.70 | 1.23 | 110.9 | [48] |
| 10 | Au-Ag/r-GO (0.1) | 4-NP | 3.47 | 34.7 | 0.042 | [49] |
| 11 | AgNPs-rGO | 2-NP | 0.44 | - | - | [50] |
| 12 | AuNPs-RGO (0.05) | 4-NP | 28.37 | 11.2 | 0.222 | [51] |
| 13 | PdNiP/RGO (3) | 4-NP | 23.51 | 7.7 | - | [52] |
| 14 | NiNPs/Silica (3) | 4-NP | 2.82 | 0.57 | - | [53] |
| 15 | RGO-ZnWO4-Fe3O4 | 4-NP | 176.8 | 353.6 | - | [54] |
| 16 | Ni/SNTs (4) | 4-NP | 2.7 | 2.6 | - | [55] |
| 17 | Ni/MC-950 | 4-NP | 2.4 | 3.4 | 1.43 | [56] |
| 18 | RGO/Ni (6.5) | 4-NP | 14.8 | 2.3 | - | [23] |
| 19 | Ni/GNS | 2-NP | 3.06 | 1.53 | 0.31 | [41] |
| 20 | Ni/HHP | 2-NP | 69.1 | 27.6 | 0.03 | [57] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gopiraman, M.; Saravanamoorthy, S.; Deng, D.; Ilangovan, A.; Kim, I.S.; Chung, I.M. Facile Mechanochemical Synthesis of Nickel/Graphene Oxide Nanocomposites with Unique and Tunable Morphology: Applications in Heterogeneous Catalysis and Supercapacitors. Catalysts 2019, 9, 486. https://doi.org/10.3390/catal9050486
Gopiraman M, Saravanamoorthy S, Deng D, Ilangovan A, Kim IS, Chung IM. Facile Mechanochemical Synthesis of Nickel/Graphene Oxide Nanocomposites with Unique and Tunable Morphology: Applications in Heterogeneous Catalysis and Supercapacitors. Catalysts. 2019; 9(5):486. https://doi.org/10.3390/catal9050486
Chicago/Turabian StyleGopiraman, Mayakrishnan, Somasundaram Saravanamoorthy, Dian Deng, Andivelu Ilangovan, Ick Soo Kim, and Ill Min Chung. 2019. "Facile Mechanochemical Synthesis of Nickel/Graphene Oxide Nanocomposites with Unique and Tunable Morphology: Applications in Heterogeneous Catalysis and Supercapacitors" Catalysts 9, no. 5: 486. https://doi.org/10.3390/catal9050486
APA StyleGopiraman, M., Saravanamoorthy, S., Deng, D., Ilangovan, A., Kim, I. S., & Chung, I. M. (2019). Facile Mechanochemical Synthesis of Nickel/Graphene Oxide Nanocomposites with Unique and Tunable Morphology: Applications in Heterogeneous Catalysis and Supercapacitors. Catalysts, 9(5), 486. https://doi.org/10.3390/catal9050486