Valorization of Olive By-Products as Substrates for the Cultivation of Ganoderma lucidum and Pleurotus ostreatus Mushrooms with Enhanced Functional and Prebiotic Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Initial Assessment of Substrates for Fungal Growth
2.2. Evaluation of TPOMW- and OLPR-Based Substrates for Mushroom Cultivation
2.3. Assessment of Nutritional Composition of Mushrooms Produced on Olive By-Products
2.4. Fourier Transform Infrared (FTIR) Analysis
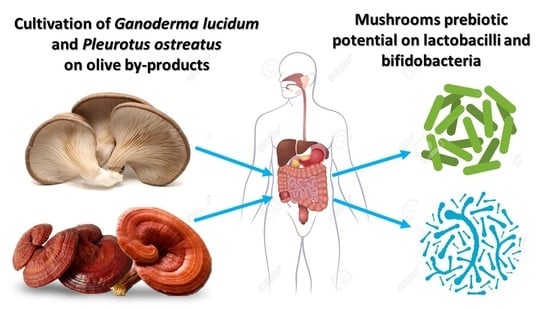
2.5. Evaluation of Prebiotic Potential of G. lucidum and P. ostreatus Mushrooms
3. Materials and Methods
3.1. Biological Material
3.2. Substrates for Fungal Growth—Determination of Mycelium Growth Rates
3.3. Mushroom Cultivation Substrates—Assessment of Production Parameters
3.4. Chemical Analysis of Mushrooms
3.5. Measurement of Total Phenolic Content and Antioxidant Activity of Mushrooms Methanolic Extracts
3.6. FTIR Analysis
3.7. Determination of Mushrooms Prebiotic Potential
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kostenidou, E.; Kaltsonoudis, C.; Tsiflikiotou, M.; Louvaris, E.; Russell, L.; Pandis, S. Olive Tree Branches Burning: A major pollution source in the Mediterranean. Geophys. Res. Abstr. 2013, 15, EGU2013-8298. [Google Scholar]
- Roig, A.; Cayuela, M.L.; Sanchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Diamantis, V.; Erguder, T.H.; Aivasidis, A.; Verstraete, W.; Voudrias, E. Wastewater disposal to landfill-sites: A synergistic solution for centralized management of olive mill wastewater and enhanced production of landfill gas. J. Environ. Manag. 2013, 128, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Barbera, A.C.; Maucieri, C.; Cavallaro, V.; Ioppolo, A.; Spagna, G. Effects of spreading olive mill wastewater on soil properties and crops, a review. Agric. Water Manag. 2013, 119, 43–53. [Google Scholar] [CrossRef]
- Chowdhury, A.K.M.M.B.; Akratos, C.S.; Vayenas, D.V.; Pavlou, S. Olive mill waste composting: A review. Int. Biodeterior. Biodegradation 2013, 85, 108–119. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Pimentel-Moral, S.; Verardo, V.; Martinez-Ferez, A. A focus on advanced physico-chemical processes for olive mill wastewater treatment. Sep. Purif. Technol. 2017, 179, 161–174. [Google Scholar] [CrossRef]
- Pulido, J.M.O. A review on the use of membrane technology and fouling control for olive mill wastewater treatment. Sci. Total Environ. 2016, 563, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Zervakis, G.I.; Koutrotsios, G. Solid state fermentation of plant residues and agro-industrial wastes for the production of medicinal mushrooms. In Medicinal Plants and Fungi: Recent Advances in Research and Development; Agrawal, D.C., Tsay, H.-S., Shyur, L.-F., Wu, Y.-C., Wang, S.-Y., Eds.; Springer Nature: Singapore, 2017; pp. 365–396. [Google Scholar] [CrossRef]
- Das, N.; Mukherjee, M. Cultivation of Pleurotus ostreatus on weed plants. Bioresour. Technol. 2007, 98, 2723–2726. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Mountzouris, K.C.; Chatzipavlidis, I.; Zervakis, G.I. Bioconversion of lignocellulosic residues by Agrocybe cylindracea and Pleurotus ostreatus mushroom fungi—Assessment of their effect on the final product and spent substrate properties. Food Chem. 2014, 161, 127–135. [Google Scholar] [CrossRef]
- Mandeel, Q.; Al-Laith, A.; Mohamed, S. Cultivation of oyster mushrooms (Pleurotus spp.) on various lignocellulosic wastes. World J. Microbiol. Biotechnol. 2005, 21, 601–607. [Google Scholar] [CrossRef]
- Membrillo, I.; Sánchez, C.; Meneses, M.; Favela, E.; Loera, O. Particle geometry affects differentially substrate composition and enzyme profiles by Pleurotus ostreatus growing on sugar cane bagasse. Bioresour. Technol. 2011, 102, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Obodai, M.; Cleland-Okine, J.; Vowotor, K. Comparative study on the growth and yield of Pleurotus ostreatus mushroom on different lignocellulosic by-products. J. Ind. Microbiol. Biotechnol. 2003, 30, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Pant, D.; Reddy, U.G.; Adholeya, A. Cultivation of oyster mushrooms on wheat straw and bagasse substrate amended with distillery effluent. World J. Microbiol. Biotechnol. 2006, 22, 267–275. [Google Scholar] [CrossRef]
- Philippoussis, A.; Zervakis, G.; Diamantopoulou, P. Bioconversion of agricultural lignocellulosic wastes through the cultivation of the edible mushrooms Agrocybe aegerita, Volvariella volvacea and Pleurotus spp. World J. Microbiol. Biotechnol. 2001, 17, 191–200. [Google Scholar] [CrossRef]
- Salmones, D.; Mata, G.; Waliszewski, K.N. Comparative culturing of Pleurotus spp. on coffee pulp and wheat straw: Biomass production and substrate biodegradation. Bioresour. Technol. 2005, 96, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Ysunza, F.; Beltrán-García, M.J.; Esqueda, M. Biodegradation of viticulture wastes by Pleurotus: A source of microbial and human food and its potential use in animal feeding. J. Agric. Food Chem. 2002, 50, 2537–2542. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Larou, E.; Mountzouris, K.; Zervakis, G.I. Detoxification of olive mill wastewater and bioconversion of olive crop residues into high-value added biomass by the choice edible mushroom Hericium erinaceus. Appl. Biochem. Biotechnol. 2016, 180, 195–209. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Kalogeropoulos, N.; Kaliora, A.C.; Zervakis, G.I. Toward an increased functionality in Oyster (Pleurotus) mushrooms produced on grape marc or olive mill wastes serving as sources of bioactive compounds. J. Agric. Food Chem. 2018, 66, 5971–5983. [Google Scholar] [CrossRef]
- Zervakis, G.; Yiatras, P.; Balis, C. Edible mushrooms from olive mill wastes. Int. Biodeterior. Biodegradation 1996, 38, 237–243. [Google Scholar] [CrossRef]
- Zervakis, G.I.; Koutrotsios, G.; Katsaris, P. Composted versus raw olive mill waste as substrates for the production of medicinal mushrooms: An assessment of selected cultivation and quality parameters. BioMed Res. Int. 2013. [Google Scholar] [CrossRef]
- Royse, D.J. A global perspective on the high five: Agaricus, Pleurotus, Lentinula, Auricularia & Flammulina. In Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products (ICMBMP8), New Delhi, India, 19–22 November 2014; pp. 1–6. [Google Scholar]
- Gargano, M.L.; van Griensven, L.J.L.D.; Isikhuemhen, O.S.; Lindequist, U.; Venturella, G.; Wasser, S.P.; Zervakis, G.I. Medicinal mushrooms: Valuable biological resources of high exploitation potential. Plant Biosyst. 2017, 151, 548–565. [Google Scholar] [CrossRef]
- Wasser, S.P. Reishi or ling zhi (Ganoderma lucidum). In Encyclopedia of Dietary Supplements, 1st ed.; Marcel Dekker, Inc.: New York, NY, USA, 2005; pp. 603–622. [Google Scholar]
- Tsapatou, A.; Mitsou, E.K.; Patsou, M.; Koutrotsios, G.; Zervakis, G.I.; Kyriacou, A. Potential prebiotic effect of Pleurotus ostreatus and Ganoderma lucidum mushrooms on human gut microbiota. In Proceedings of the Gut Microbiota for Health World Summit 2015, Barcelona, Spain, 14–15 March 2015; p. 18. [Google Scholar]
- Yamin, S.; Shuhaimi, M.; Arbakariya, A.; Fatimah, A.B.; Khalilah, A.K.; Anas, O.; Yazid, A.M. Effect of Ganoderma lucidum polysaccharides on the growth of Bifidobacterium spp. as assessed using real-time PCR. Int. Food Res. J. 2012, 19, 1199–1205. [Google Scholar]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [PubMed]
- Camilli, G.; Tabouret, G.; Quintin, J. The complexity of fungal β-glucan in health and disease: Effects on the mononuclear phagocyte system. Front. Immunol. 2018, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Lin, C.-S.; Lu, C.-C.; Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Tseng, S.-F.; Wu, T.-R.; Chen, Y.-Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.C.; Antunes, M.B.; Rodrigues, D.; Sousa, S.; Amorim, M.; Barroso, M.F.; Carvalho, A.; Ferrador, S.M.; Gomes, A.M. Use of coffee by-products for the cultivation of Pleurotus citrinopileatus and Pleurotus salmoneo-stramineus and its impact on biological properties of extracts thereof. Int. J. Food Sci. Technol. 2018, 53, 1914–1924. [Google Scholar] [CrossRef]
- Khan, I.; Huang, G.; Li, X.; Leong, W.; Xia, W.; Hsiao, W.L.W. Mushroom polysaccharides from Ganoderma lucidum and Poria cocos reveal prebiotic functions. J. Funct. Foods 2018, 41, 191–201. [Google Scholar] [CrossRef]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compost Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Avni, S.; Ezove, N.; Hanani, H.; Yadid, I.; Karpovsky, M.; Hayby, H.; Gover, O.; Hadar, Y.; Schwartz, B.; Danay, O. Olive mill waste enhances α-glucan content in the edible mushroom Pleurotus eryngii. Int. J. Mol. Sci. 2017, 18, 1564. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Kalogeropoulos, N.; Stathopoulos, P.; Kaliora, A.; Zervakis, G.I. Bioactive compounds and antioxidant activity exhibit high intraspecific variability in Pleurotus ostreatus mushrooms and correlate well with cultivation performance parameters. World J. Microbiol. Biotechnol. 2017, 33, 98. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, C.; Wen, H.A.; Liu, X. Development of Ling-zhi industry in China–emanated from the artificial cultivation in the Institute of Microbiology, Chinese Academy of Sciences (IMCAS). Mycology 2016, 7, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Jiang, J.; He, C.; Liu, M.; Liu, D. Preliminary researches on high-yield cultivation techniques of Ganoderma. Hunan Agric. Sci. 2003, 6, 5658. [Google Scholar]
- Zhou, X.-W.; Su, K.-Q.; Zhang, Y.-M. Applied modern biotechnology for cultivation of Ganoderma and development of their products. Appl. Microbiol. Biotechnol. 2012, 93, 941–963. [Google Scholar] [CrossRef] [PubMed]
- Peksen, A.; Yakupoglu, G. Tea waste as a supplement for the cultivation of Ganoderma lucidum. World J. Microbiol. Biotechnol. 2009, 25, 611–618. [Google Scholar] [CrossRef]
- Yang, F.-C.; Hsieh, C.; Chen, H.-M. Use of stillage grain from a rice-spirit distillery in the solid state fermentation of Ganoderma lucidum. Process Biochem. 2003, 39, 21–26. [Google Scholar] [CrossRef]
- Ji, H.; Wang, Q.; Wang, H.; Chen, W.; Zhu, C.; Hou, H.; Zhang, Z. A fundamental research of mushroom cultivation using maize straw. Edible Fungi China 2001, 20, 10–17. [Google Scholar]
- Aggoun, M.; Arhab, R.; Cornu, A.; Portelli, J.; Barkat, M.; Graulet, B. Olive mill wastewater microconstituents composition according to olive variety and extraction process. Food Chem. 2016, 209, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Ntougias, S.; Gaitis, F.; Katsaris, P.; Skoulika, S.; Iliopoulos, N.; Zervakis, G.I. The effects of olives harvest period and production year on olive mill wastewater properties—evaluation of Pleurotus strains as bioindicators of the effluent’s toxicity. Chemosphere 2013, 92, 399–405. [Google Scholar] [CrossRef]
- Ruiz-Rodriguez, A.; Soler-Rivas, C.; Polonia, I.; Wichers, H.J. Effect of olive mill waste (OMW) supplementation to oyster mushrooms substrates on the cultivation parameters and fruiting bodies quality. Int. Biodeterior. Biodegradation 2010, 64, 638–645. [Google Scholar] [CrossRef]
- Fernandes, Â.; Barros, L.; Martins, A.; Herbert, P.; Ferreira, I.C. Nutritional characterisation of Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm. produced using paper scraps as substrate. Food Chem. 2015, 169, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Manzi, P.; Gambelli, L.; Marconi, S.; Vivanti, V.; Pizzoferrato, L. Nutrients in edible mushrooms: An inter-species comparative study. Food Chem. 1999, 65, 477–482. [Google Scholar] [CrossRef]
- Wang, D.; Sakoda, A.; Suzuki, M. Biological efficiency and nutritional value of Pleurotus ostreatus cultivated on spent beer grain. Bioresour. Technol. 2001, 78, 293–300. [Google Scholar] [CrossRef]
- Atila, F.; Tüzel, Y.; Faz Cano, A.; Fernandez, J.A. Effect of different lignocellulosic wastes on Hericium americanum yield and nutritional characteristics. J. Sci. Food Agric. 2017, 97, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Isikhuemhen, O.S.; Mikiashvili, N.A.; Kelkar, V. Application of solid waste from anaerobic digestion of poultry litter in Agrocybe aegerita cultivation: Mushroom production, lignocellulolytic enzymes activity and substrate utilization. Biodegradation 2009, 20, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Uhart, M.; Piscera, J.M.; Albert, E. Utilization of new naturally occurring strains and supplementation to improve the biological efficiency of the edible mushroom Agrocybe cylindracea. J. Ind. Microbiol. Biotechnol. 2008, 35, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Sari, M.; Prange, A.; Lelley, J.I.; Hambitzer, R. Screening of beta-glucan contents in commercially cultivated and wild growing mushrooms. Food Chem. 2017, 216, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Malavazi, I.; Goldman, G.H.; Brown, N.A. The importance of connections between the cell wall integrity pathway and the unfolded protein response in filamentous fungi. Brief. Funct. Genomics 2014, 13, 456–470. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.; Yang, F.-C. Reusing soy residue for the solid-state fermentation of Ganoderma lucidum. Bioresour. Technol. 2004, 91, 105–109. [Google Scholar] [CrossRef]
- Shi, M.; Yang, Y.; Guan, D.; Wang, Y.; Zhang, Z. Evaluation of solid-state fermentation by Ganoderma lucidum using soybean curd residue. Food Bioproc. Tech. 2013, 6, 1856–1867. [Google Scholar] [CrossRef]
- Ćilerdžić, J.; Vukojević, J.; Stajić, M.; Stanojković, T.; Glamočlija, J. Biological activity of Ganoderma lucidum basidiocarps cultivated on alternative and commercial substrate. J. Ethnopharmacol. 2014, 155, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wily & Sons Ltd.: Chichester, UK, 2001. [Google Scholar]
- Synytsya, A.; Novak, M. Structural analysis of glucans. Ann. Transl. Med. 2014, 2, 17. [Google Scholar] [PubMed]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Mohd Hamim, H.M.; Shuhaimi, M.; Yazid, A.M.; Ali, A.M.; Anas, O.M.; Asilah, A.T.; Wahab, M.N.; Shukor, M.Y.A. Growth of probiotic bacteria in trypticase phytone yeast medium supplemented with crude polysaccharides from Ganoderma lucidum. Malays. J. Microbiol. 2010, 6, 47–56. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lin, Y.S.; Lin, K.L.; Lu, Y.L.; Chen, C.H.; Chien, M.Y.; Shang, H.F.; Lin, S.Y.; Hou, W.C. Effects of hot-water extracts from Ganoderma lucidum residues and solid-state fermentation residues on prebiotic and immune-stimulatory activities in vitro and the powdered residues used as broiler feed additives in vivo. Bot. Stud. 2015, 56, 17. [Google Scholar] [CrossRef] [PubMed]
- Meneses, M.E.; Martínez-Carrera, D.; Torres, N.; Sánchez-Tapia, M.; Aguilar-López, M.; Morales, P.; Sobal, M.; Bernabé, T.; Escudero, H.; Granados-Portillo, O.; et al. Hypocholesterolemic properties and prebiotic effects of Mexican Ganoderma lucidum in C57BL/6 mice. PLoS ONE 2016, 11, e0159631. [Google Scholar] [CrossRef]
- Saidou, C.; Tchatchueng, J.B.; Ndjouenkeu, R.; Roux, D. Extraction and partial characterisation of hydrocolloid gums from some African legumes. Int. J. Food Eng. 2011, 7, 15. [Google Scholar] [CrossRef]
- Zervakis, G.; Philippoussis, A.; Ioannidou, S.; Diamantopoulou, P. Mycelium growth kinetics and optimal temperature conditions for the cultivation of edible mushroom species on lignocellulosic substrates. Folia Microbiol. 2001, 46, 231–234. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Manzi, P.; Marconi, S.; Aguzzi, A.; Pizzoferrato, L. Commercial mushrooms: Nutritional quality and effect of cooking. Food Chem. 2004, 84, 201–206. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Yanni, A.E.; Koutrotsios, G.; Aloupi, M. Bioactive microconstituents and antioxidant properties of wild edible mushrooms from the island of Lesvos, Greece. Food Chem. Toxicol. 2013, 55, 378–385. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of pigment and flavanol content with antioxidant properties in selected aged regional wines from Greece. J. Food Compost Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Roy, D. Media for the isolation and enumeration of bifidobacteria in dairy products. Int. J. Food Microbiol. 2001, 69, 167–182. [Google Scholar] [CrossRef]






| Ganoderma lucidum | ||||||
| BS | BS:OLPR 3:1 | BS:OLPR 1:1 | BS:OLPR 1:3 | BS:TPOMW 3:1 | BS:TPOMW 1:1 | |
| Incubation period (days) | 43.00 ± 0.00a | 43.00 ± 0.00a | 26.33 ± 0.67c | 31.25 ± 0.63b | 33.00 ± 1.16b | 26.33 ± 0.67c |
| Earliness (days) | 45.75 ± 0.25b | 45.00 ± 3.68b | 52.75 ± 4.50ab | 63.67 ± 15.50ab | 77.00 ± 18.54a | 83.50 ± 19.50a |
| Total yield (g) | 275.22 ± 14.48a | 193.42 ± 13.52b | 61.46 ± 10.8c | 19.10 ± 8.3d | 36.10 ± 14.47cd | 61.46 ± 10.84c |
| Biological efficiency (%) | 61.24 ± 3.22a | 40.24 ± 2.81b | 20.52 ± 3.62c | 4.54 ± 1.97d | 12.05 ± 4.83c | 20.52 ± 3.62c |
| Pleurotus ostreatus | ||||||
| WS | WS:OLPR 3:1 | WS:OLPR 1:1 | WS:OLPR 1:3 | WS:TPOMW 3:1 | WS:TPOMW 1:1 | |
| Incubation period (days) | 26.00 ± 0.00c | 28.00 ± 0.33b | 30.67 ± 2.33ab | 36.47 ± 2.19a | 25.00 ± 0.00d | 27.00 ± 0.00b |
| Earliness (days) | 40.67 ± 4.81bc | 37.25 ± 1.53c | 41.27 ± 1.16bc | 46.00 ± 2.00b | 40.50 ± 1.44bc | 58.67 ± 5.55a |
| Total yield (g) | 215.87 ± 15.43a | 263.84 ± 46.40a | 255.72 ± 28.04a | 134.87 ± 4.82b | 220.75 ± 3.17a | 256.87 ± 44.34a |
| Biological efficiency (%) | 77.26 ± 5.52a | 82.60 ± 14.53a | 56.79 ± 6.23b | 39.73 ± 1.42c | 73.68 ± 1.06a | 71.33 ± 12.31ab |
| Ganoderma lucidum | ||||||
| BS | BS:OLPR 3:1 | BS:OLPR 1:1 | BS:OLPR 1:3 | BS:TPOMW 3:1 | BS:TPOMW 1:1 | |
| Ash | 3.10 ± 0.08c | 3.08 ± 0.12c | 3.41 ± 0.33c | 5.20 ± 0.32a | 3.68 ± 0.07bc | 4.26 ± 0.18b |
| Crude fiber | 47.93 ± 1.86b | 62.48 ± 1.18a | 43.80 ± 2.02b | 21.57 ± 9.66c | 52.89 ± 3.77ab | 49.34 ± 1.56b |
| Crude fat | 2.21 ± 0.08a | 2.45 ± 0.05a | 2.03 ± 0.49a | 2.04 ± 0.50a | 1.56 ± 0.52a | 1.10 ± 0.12a |
| Crude protein | 16.84 ± 0.62bc | 17.06 ± 0.15bc | 15.28 ± 0.55c | 23.25 ± 4.08abc | 18.85 ± 0.55ab | 22.21 ± 0.79a |
| T. carbohydrates | 77.86 ± 1.05a | 77.41 ± 2.07a | 79.28 ± 3.47a | 69.52 ± 3.29b | 75.92 ± 1.85a | 72.44 ± 1.38ab |
| Gross energy | 399 ± 13a | 400 ± 17a | 397 ± 4a | 389 ± 11a | 393 ± 2a | 388 ± 3a |
| α-glucan | 2.09 ± 0.48b | 1.68 ± 0.68b | 6.84 ± 1.99a | 6.22 ± 2.27a | 3.08 ± 1.38ab | 3.87 ± 0.30ab |
| β-glucan | 35.83 ± 2.05a | 43.10 ± 6.38a | 35.06 ± 5.14a | 31.27 ± 6.19a | 34.72 ± 1.03a | 32.87 ± 2.00a |
| Total phenolics | 3.25 ± 0.09b | 4.23 ± 0.27a | 3.89 ± 1.15ab | 4.02 ± 0.51a | 2.98 ± 0.31b | 2.99 ± 0.32b |
| Antiradical activity | 8.49 ± 1.32a | 9.56 ± 0.41a | 8.46 ± 1.05a | 10.41 ± 1.37a | 7.18 ± 2.78a | 6.37 ± 0.72a |
| Reducing power | 12.99 ± 1.95b | 13.96 ± 0.41b | 18.90 ± 0.89a | 13.76 ± 4.88ab | 12.93 ± 3.82b | 13.26 ± 3.93b |
| Pleurotus ostreatus | ||||||
| WS | WS:OLPR 3:1 | WS:OLPR 1:1 | WS:OLPR 1:3 | WS:TPOMW 3:1 | WS:TPOMW 1:1 | |
| Ash | 8.49 ± 0.82a | 7.95 ± 0.10a | 6.49 ± 0.14b | 6.32 ± 0.45b | 8.99 ± 0.03a | 9.42 ± 1.90a |
| Crude fiber | 18.99 ± 1.97a | 17.16 ± 1.24a | 16.54 ± 1.94a | 14.01 ± 1.34ab | 15.47 ± 1.13ab | 12.97 ± 2.44b |
| Crude fat | 2.54 ± 0.17a | 2.47 ± 0.14a | 1.87 ± 0.04b | 1.62 ± 0.21b | 2.74 ± 0.17a | 2.70 ± 0.21a |
| Crude protein | 15.22 ± 1.29c | 16.00 ± 0.37c | 19.88 ± 2.34a | 21.54 ± 0.24a | 17.08 ± 0.58b | 19.32 ± 0.24ab |
| T. carbohydrates | 73.75 ± 2.81a | 73.58 ± 4.27a | 71.76 ± 0.87a | 70.52 ± 1.46a | 71.19 ± 0.98a | 68.56 ± 3.64a |
| Gross energy | 379 ± 5a | 381 ± 14a | 383 ± 2a | 383 ± 9a | 378 ± 4a | 376 ± 9a |
| α-glucan | 8.75 ± 0.37a | 7.25 ± 1.98a | 6.32 ± 2.07a | 5.69 ± 2.40a | 6.00 ± 1.14a | 2.17 ± 0.58b |
| β-glucan | 30.64 ± 2.45a | 28.02 ± 1.61a | 28.87 ± 3.45a | 25.58 ± 0.21a | 31.49 ± 0.43a | 29.56 ± 2.42a |
| Total phenolics | 2.01 ± 0.11c | 3.09 ± 0.34a | 2.73 ± 0.17ab | 2.57 ± 0.27b | 2.81 ± 0.24a | 2.99 ± 0.34a |
| Antiradical activity | 2.49 ± 0.14b | 3.79 ± 0.47ab | 2.81 ± 0.57b | 5.59 ± 1.21a | 3.27 ± 0.43ab | 4.68 ± 0.67a |
| Reducing power | 5.78 ± 0.88a | 6.69 ± 1.27a | 6.85 ± 1.39a | 5.55 ± 0.22a | 3.47 ± 0.27b | 3.94 ± 0.04b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koutrotsios, G.; Patsou, M.; Mitsou, E.K.; Bekiaris, G.; Kotsou, M.; Tarantilis, P.A.; Pletsa, V.; Kyriacou, A.; Zervakis, G.I. Valorization of Olive By-Products as Substrates for the Cultivation of Ganoderma lucidum and Pleurotus ostreatus Mushrooms with Enhanced Functional and Prebiotic Properties. Catalysts 2019, 9, 537. https://doi.org/10.3390/catal9060537
Koutrotsios G, Patsou M, Mitsou EK, Bekiaris G, Kotsou M, Tarantilis PA, Pletsa V, Kyriacou A, Zervakis GI. Valorization of Olive By-Products as Substrates for the Cultivation of Ganoderma lucidum and Pleurotus ostreatus Mushrooms with Enhanced Functional and Prebiotic Properties. Catalysts. 2019; 9(6):537. https://doi.org/10.3390/catal9060537
Chicago/Turabian StyleKoutrotsios, Georgios, Marianna Patsou, Evdokia K. Mitsou, Georgios Bekiaris, Maria Kotsou, Petros A. Tarantilis, Vasiliki Pletsa, Adamantini Kyriacou, and Georgios I. Zervakis. 2019. "Valorization of Olive By-Products as Substrates for the Cultivation of Ganoderma lucidum and Pleurotus ostreatus Mushrooms with Enhanced Functional and Prebiotic Properties" Catalysts 9, no. 6: 537. https://doi.org/10.3390/catal9060537
APA StyleKoutrotsios, G., Patsou, M., Mitsou, E. K., Bekiaris, G., Kotsou, M., Tarantilis, P. A., Pletsa, V., Kyriacou, A., & Zervakis, G. I. (2019). Valorization of Olive By-Products as Substrates for the Cultivation of Ganoderma lucidum and Pleurotus ostreatus Mushrooms with Enhanced Functional and Prebiotic Properties. Catalysts, 9(6), 537. https://doi.org/10.3390/catal9060537