Oxidative Thermal Sintering and Redispersion of Rh Nanoparticles on Supports with High Oxygen Ion Lability
Abstract
:1. Introduction
2. Results and Discussion
2.1. Materials Characterization
2.1.1. Textural Characteristics
2.1.2. Reducibility Characteristics
2.1.3. Structural and Morphological Characteristics
2.2. Sintering Behavior
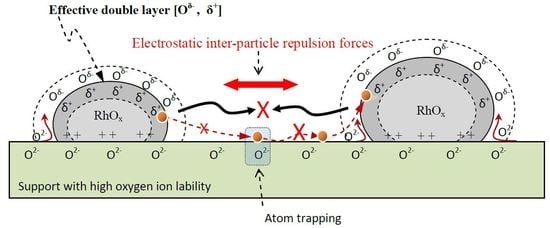
2.3. Mechanistic Implications of Sinter-Resistant and Redispersion Phenomena
3. Experimental
3.1. Preparation and Aging of Catalysts
3.2. Characterization Methods
4. Conclusions
- γ-Al2O3 provided little or no resistance to sintering, leading to ~50% Rh particle growth at 750 °C after 2 h and ~150% at 850 °C after two additional hours.
- High resistance to sintering and even redispersion occurred on ACZ and CZ, characterized respectively by moderate and high values of labile lattice oxygen capacity (~101 and 557 μmol O2 g−1, respectively); the higher the OSC of the support, the greater the extent of particle redispersion—which increased with increasing sintering temperature.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Flytzani-Stephanopoulos, M.; Gates, B.C. Atomically dispersed supported metal catalysts. Ann. Rev. Chem. Biomol. Eng. 2012, 3, 545–574. [Google Scholar] [CrossRef]
- Pliangos, A.; Yentekakis, I.V.; Papadakis, V.G.; Vayenas, C.G.; Verykios, X.E. Support-induced promotional effects on the activity of automotive exhaust catalysts: 1. The case of oxidation of light hydrocarbons (C2H4). Appl. Catal. B Environ. 1997, 14, 161–173. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Pliangos, C.A.; Yentekakis, I.V.; Verykios, X.E.; Vayenas, C.G. Development of high performance, Pd-based, three way catalysts. Catal. Today 1996, 29, 71–75. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Pliangos, C.A.; Papadakis, V.G.; Verykios, X.E.; Vayenas, C.G. Support and NEMCA induced promotional effects on the activity of automotive exhaust catalysts. Stud. Surf. Sci. Catal. 1995, 96, 375–385. [Google Scholar] [CrossRef]
- Konsolakis, M.; Drosou, C.; Yentekakis, I.V. Support mediated promotional effects of rare earth oxides (CeO2 and La2O3) on N2O decomposition and N2O reduction by CO and C3H6 over Pt/Al2O3 structured catalysts. Appl. Catal. B 2012, 123–124, 405–413. [Google Scholar] [CrossRef]
- Papavasiliou, A.; Tsetsekou, A.; Matsuka, V.; Konsolakis, M.; Yentekakis, I.V. An investigation of the role of Zr and La dopants into Ce1-x-yZrxLayOδ enriched γ-Al2O3 TWC washcoats. Appl. Catal. A 2010, 382, 73–84. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Kampouri, S.; Betsi-Argyropoulou, I.; Panagiotopoulou, P.; Taylor, M.J.; Kyriakou, G.; Lambert, R.M. Ir-catalyzed Nitrous oxide (N2O) decomposition: Effect of the Ir particle size and metal-support interactions. Catal. Lett. 2018, 148, 341–347. [Google Scholar] [CrossRef]
- Nicole, J.; Tsiplakides, D.; Pliangos, C.; Verykios, X.E.; Comninellis, C.; Vayenas, C.G. Electrochemical promotion and metal-support interactions. J. Catal. 2001, 204, 23–34. [Google Scholar] [CrossRef]
- Vernoux, P.; Lizarraga, L.; Tsampas, M.N.; Sapountzi, F.M.; De Lucas-Consuegra, A.; Valverde, J.-L.; Souentie, S.; Vayenas, C.G.; Tsiplakides, D.; Balomenou, S.; et al. Ionically Conducting Ceramics as Active Catalyst Supports. Chem. Rev. 2013, 113, 8192–8260. [Google Scholar] [CrossRef]
- Datye, A.; Wang, Y. Atom trapping: A novel approach to generate thermally stable and regenerable single-atom catalysts. Natl. Sci. Rev. 2018, 5, 630–632. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Xiong, H.; DeLaRiva, A.T.; Peterson, E.J.; Pham, H.; Challa, S.R.; Qi, G.; Oh, S.; Wiebenga, M.H.; Hernandez, X.I.P.; et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grillo, F.; Van Bui, H.; Moulijn, J.A.; Kreutzer, M.T.; van Ommen, R. Understanding and controlling the aggregative growth of platinum nanoparticles in atomic layer deposition: An avenue to size selection. J. Phys. Chem. Lett. 2017, 8, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Grillo, F.; Van Bui, H.; La Zara, D.; Aarnink, A.I.; Kovalgin, A.Y.; Kooyman, P.; Kreutzer, M.T.; van Ommen, R. From single atoms to nanoparticles: Autocatalysis and metal aggregation in atomic layer deposition of Pt on TiO2 nanopowder. Small 2018, 14, 1800765. [Google Scholar] [CrossRef] [PubMed]
- Moulijn, J.A.; van Diepen, A.E.; Kapteijn, F. Catalyst deactivation: Is it predictable? What to do? Appl. Catal. A 2001, 212, 3–16. [Google Scholar] [CrossRef]
- Hu, S.; Li, W.-X. Influence of particle size distribution on lifetime and thermal stability of Ostwald ripening of supported particles. ChemCatChem 2018, 10, 1–9. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, P.; Cao, Z.; Campbell, C.T.; Xia, Y. The physical chemistry and materials science behind sinter-resistant catalysts. Chem. Soc. Rev. 2018, 47, 4314–4331. [Google Scholar] [CrossRef] [PubMed]
- Goodman, E.D.; Schwalbe, J.A.; Cargnello, M. Mechanistic understanding and rational design of sinter-resistant heterogeneous catalysts. ACS Catal. 2017, 7, 7156–7173. [Google Scholar] [CrossRef]
- Hansen, T.W.; DeLaRiva, A.T.; Challa, S.R.; Datye, A.K. Sintering of Catalytic Nanoparticles: Particle Migration or Ostwald Ripening? Acc. Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Simonsen, S.B.; Chorkendorff, I.; Dahl, S.; Skoglundh, M.; Sehested, J.; Helveg, S. Direct observations of oxygen-induced platinum nanoparticle ripening studied by in situ TEM. J. Am. Chem. Soc. 2010, 132, 7968–7975. [Google Scholar] [CrossRef] [PubMed]
- DeLaRiva, A.T.; Hansen, T.W.; Challa, S.R.; Datye, A.K. In situ Transmission Electron Microscopy of catalyst sintering. J. Catal. 2013, 308, 291–305. [Google Scholar] [CrossRef]
- Fiedorow, R.M.J.; Chahar, B.S.; Wanke, S.E. The sintering of supported metal catalysts. II. Comparison of sintering rates of supported Pt, Ir, and Rh Catalysts in hydrogen and oxygen. J. Catal. 1978, 51, 193–202. [Google Scholar] [CrossRef]
- Nagai, Y.; Hirabayashi, T.; Dohmae, K.; Takagi, N.; Minami, T.; Shinjoh, H.; Matsumoto, S. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide-support interaction. J. Catal. 2006, 242, 103–109. [Google Scholar] [CrossRef]
- Hatanaka, M.; Takahashi, N.; Tanabe, T.; Nagai, Y.; Dohmae, K.; Aoki, Y.; Yoshida, T.; Shinjoh, H. Ideal Pt loading for a Pt/CeO2-based catalyst stabilized by Pt-O-Ce bond. Appl. Catal. B 2010, 99, 336–342. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Panagiotopoulou, P.; Kampouri, S.; Taylor, M.J.; Kyriakou, G.; Lambert, R.M. Stabilization of catalyst particles against sintering on oxide supports with high oxygen ion lability exemplified by Ir-catalysed decomposition of N2O. Appl. Catal. B 2016, 192, 357–364. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Panagiotopoulou, P.; Katsoni, A.; Diamadopoulos, E.; Mantzavinos, D.; Delimitis, A. Dry reforming of methane: Catalytic performance and stability of Ir catalysts supported on γ-Al2O3, Zr0.92Y0.08O2-δ (YSZ) or Ce0.9Gd0.1O2-δ (GDC) supports. Top. Catal. 2015, 58, 1228–1241. [Google Scholar] [CrossRef]
- Nishihata, Y.; Mizuki, J.; Akao, T.; Tanaka, H.; Uenishi, M.; Kimura, M.; Okamoto, T.; Hamada, N. Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 2002, 418, 164–167. [Google Scholar] [CrossRef]
- Morgan, K.; Goguet, A.; Hardacre, C. Metal redispersion strategies for recycling of supported metal catalysts: A perspective. ACS Catal. 2015, 5, 3430–3445. [Google Scholar] [CrossRef]
- Nagai, Y.; Dohmae, K.; Ikeda, Y.; Takagi, N.; Tanabe, T.; Hara, N.; Guilera, G.; Pascarelli, S.; Newton, M.A.; Kuno, O.; et al. In situ redispersion of platinum autoexhaust catalysts: An on-line approach to increasing catalyst lifetimes? Angew. Chem. Int. Ed. 2008, 47, 9303–9306. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Nagai, Y.; Dohmae, K.; Sobukawa, H.; Shinjoh, H. Sintering and redispersion behavior of Pt on Pt/MgO. J. Catal. 2008, 257, 117–124. [Google Scholar] [CrossRef]
- Tanabe, T.; Morikawa, A.; Hatanaka, M.; Takahashi, N.; Nagai, Y.; Sato, A.; Kuno, O.; Suzuki, H.; Shinjoh, H. The interaction between supported Rh- and Nd2O3-enriched surface layer on ZrO2 for Rh sintering suppression. Catal. Today 2012, 184, 219–226. [Google Scholar] [CrossRef]
- Morikawa, A.; Tanabe, T.; Hatanaka, M.; Takahashi, N.; Sato, A.; Kuno, O.; Suzuki, H.; Shinjoh, H. Inhibition of Rh sintering and improved reducibility of Rh on ZrO2 nanocomposite with an Al2O3 diffusion barrier. Appl. Catal. A 2015, 493, 33–39. [Google Scholar] [CrossRef]
- Cao, Y.; Ran, R.; Wu, X.-Y.; Wu, X.-D.; Wan, J.; Weng, D. Ageing resistance of rhodium supported on CeO2-ZrO2 and ZrO2: Rhodium nanoparticle structure and Rh-support interaction under diverse ageing atmosphere. Catal. Today 2017, 281, 490–499. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, R.; Cao, Y.; Wu, X.; Weng, D.; Fan, J.; Wu, X. Insight into the effects of different ageing protocols on Rh/Al2O3 catalyst. Appl. Surf. Sci. 2014, 308, 230–236. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special references to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Lan, L.; Chen, S.; Zhao, M.; Gong, M.; Chen, Y. The effect of synthesis method on the properties and catalytic performance of Pd/Ce0.5Zr0.5O2-Al2O3 theree-way catalyst. J. Mol. Catal. A Chem. 2014, 394, 10–21. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, X.; Li, S.; Xiong, L.; Yan, S.; Wang, J.; Chen, Y. Promotional effect of alkyl acids on thermal stability of nanostructured CeO-ZrO2-Y2O3-La2O3 and its application in automotive three-way catalysts. Mater. Sci. Eng. B 2017, 225, 10–19. [Google Scholar] [CrossRef]
- Chen, H.; Ye, Z.; Cui, X.; Shi, J.; Yan, D. A novel mesostructured alumina-ceria-zirconia tri-component nanocomposite with high thermal stability and its trhree-way catalysis. Microporous Mesoporous Mater. 2011, 143, 368–374. [Google Scholar] [CrossRef]
- Fornasiero, P.; Di Monte, R.; Ranga Rao, G.; Kaspar, J.; Meriani, S.; Trovarelli, A.; Graziani, M. Rh-Loaded CeO2-ZrO2 Solid-Solutions as Highly Efficient Oxygen Exchangers: Dependence of the Reduction Behavior and the Oxygen Storage Capacity on the Structural-Properties. J. Catal. 1995, 151, 168–177. [Google Scholar] [CrossRef]
- Ozawa, M.; Takahashi-Morita, M.; Kobayashi, K.; Haneda, M. Core-shell type ceria zirconia support for platinum and rhodium three way catalysts. Catal. Today 2017, 281, 482–489. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Hatzisymeon, M.; Betsi-Argyropoulou, I.; Botzolaki, G.; Kiousi, K.; Kontarides, D.I.; Taylor, M.J.; Parlett, C.M.A.; Osatiashtiani, A.; et al. Effect of support oxygen storage capacity on the catalytic performance of Rh nanoparticles for CO2 reforming of methane. Appl. Catal. B 2019, 243, 490–501. [Google Scholar] [CrossRef]
- Morikawa, A.; Suzuki, T.; Kanazawa, T.; Kikuta, K.; Suda, A.; Shinjo, H. A new concept in high performance ceria–zirconia oxygen storage capacity material with Al2O3 as a diffusion barrier. Appl. Catal. B Environ. 2008, 78, 210–221. [Google Scholar] [CrossRef]
- Silva, F.A.; Martinez, D.S.; Ruiz, J.A.C.; Mattos, L.V.; Hori, C.H.; Noronha, F.B. The effect of the use of cerium-doped alumina on the performance of Pt/CeO2/Al2O3 and Pt/CeZrO2/Al2O3 catalysts on the partial oxidation of methane. Appl. Catal. A Gen. 2008, 335, 145–152. [Google Scholar] [CrossRef]
- Vayenas, C.G.; Brosda, S.; Pliangos, C. The double-layer approach to promotion, electrocatalysis, electrochemical promotion, and metal–support interactions. J. Catal. 2003, 216, 487–504. [Google Scholar] [CrossRef]
- Vayenas, C.G.; Bebelis, S.; Yentekakis, I.V.; Lintz, H.-G. Non-faradaic electrochemical modification of catalytic activity: A status report. Catal. Today 1992, 11, 303–438. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Crookes, W. The volatility of metals of the platinum group. Proc. R. Soc. A Math. Phys. Eng. Sci. 1912, 86. [Google Scholar] [CrossRef]
- Carol, L.A.; Mann, G.S. High-temperature oxidation of rhodium. Oxid. Met. 1990, 34, 1–12. [Google Scholar] [CrossRef]
- Powell, A.R. Behaviour of the platinum metals at high temperatures. Platin. Met. Rev. 1958, 2, 96–98. [Google Scholar]
- Li, P.; Chen, X.; Li, Y.; Schwank, J.W. A review on oxygen storage capacity of CeO2-based materials: Influence factors, measurement techniques, and applications in reactions related to catalytic automotive emissions control. Catal. Today 2019, 327, 90–115. [Google Scholar] [CrossRef]
- Yang, T.; Xia, D. Self-combustion synthesis and oxygen storage properties of mesoporous gadolinia-doped ceria nanotubes. Mater. Chem. Phys. 2010, 123, 816–820. [Google Scholar] [CrossRef]








| Supports and Catalysts | Chemical Formula | Rh Loading (wt %) a | SBET (m2 g−1) | Total Pore Volume (cm3 g−1) | Average Pore Size Diameter (nm) | OSC (μmol O2 g−1) | Experimental vs. Theoretical O2 Bound to Rh (μmol O2 g−1) c |
|---|---|---|---|---|---|---|---|
| γ-Al2O3 | γ-Al2O3 | 178 | 0.60 | 13.5 | 0 | ||
| Rh/γ-Al2O3 | Rh/γ-Al2O3 | 1.0 | 160 | 0.57 | 14.2 | 69 b | 69 vs. 73 |
| ACZ | 80 wt %Al2O3–20 wt %Ce0.5Zr0.5O2-δ | 149 | 0.29 | 7.9 | 101 | ||
| Rh/ACZ | Rh/(80 wt %Al2O3–20 wt %Ce0.5Zr0.5O2-δ) | 0.8 | 136 | 0.28 | 8.2 | 146 b | 45 vs. 58.4 |
| CZ | Ce0.5Zr0.5O2-δ | 22 | 0.05 | 9.2 | 557 | ||
| Rh/CZ | Rh/Ce0.5Zr0.5O2-δ | 0.8 | 17 | 0.05 | 9.3 | 589 b | 32 vs. 58.4 |
| Catalysts | Mean Rh Particle Size (nm) | |||||
|---|---|---|---|---|---|---|
| Fresh | Sinter #1 | Sinter #2 | ||||
| H2-Chem | HRTEM | H2-Chem | HRTEM | H2-Chem | HRTEM | |
| Rh/γ-Al2O3 | 1.2 | 1.3 ± 0.4 | 1.6 | 1.6 ± 0.3 | 2.6 | 1.6 ± 0.3 |
| Rh/ACZ | 1.8 | 1.5 ± 0.5 | 1.4 | 2.0 ± 0.8 | 1.7 | 1.5 ± 0.4 |
| Rh/CZ | 5.0 | 5.1 ± 1.7 | 2.1 | 2.5 ± 0.7 | 2.2 | 2.0 ± 0.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goula, G.; Botzolaki, G.; Osatiashtiani, A.; Parlett, C.M.A.; Kyriakou, G.; Lambert, R.M.; Yentekakis, I.V. Oxidative Thermal Sintering and Redispersion of Rh Nanoparticles on Supports with High Oxygen Ion Lability. Catalysts 2019, 9, 541. https://doi.org/10.3390/catal9060541
Goula G, Botzolaki G, Osatiashtiani A, Parlett CMA, Kyriakou G, Lambert RM, Yentekakis IV. Oxidative Thermal Sintering and Redispersion of Rh Nanoparticles on Supports with High Oxygen Ion Lability. Catalysts. 2019; 9(6):541. https://doi.org/10.3390/catal9060541
Chicago/Turabian StyleGoula, Grammatiki, Georgia Botzolaki, Amin Osatiashtiani, Christopher M. A. Parlett, Georgios Kyriakou, Richard M. Lambert, and Ioannis V. Yentekakis. 2019. "Oxidative Thermal Sintering and Redispersion of Rh Nanoparticles on Supports with High Oxygen Ion Lability" Catalysts 9, no. 6: 541. https://doi.org/10.3390/catal9060541
APA StyleGoula, G., Botzolaki, G., Osatiashtiani, A., Parlett, C. M. A., Kyriakou, G., Lambert, R. M., & Yentekakis, I. V. (2019). Oxidative Thermal Sintering and Redispersion of Rh Nanoparticles on Supports with High Oxygen Ion Lability. Catalysts, 9(6), 541. https://doi.org/10.3390/catal9060541