Crystal Structure and Supramolecular Architecture of Antiallergic Diphenylene Diethyl Dioxalamates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crystallization of Compounds 1-3
2.2. X-ray Diffraction
2.3. Computational Details
3. Results and Discussion
3.1. Crystal Structure of Compounds 1-3
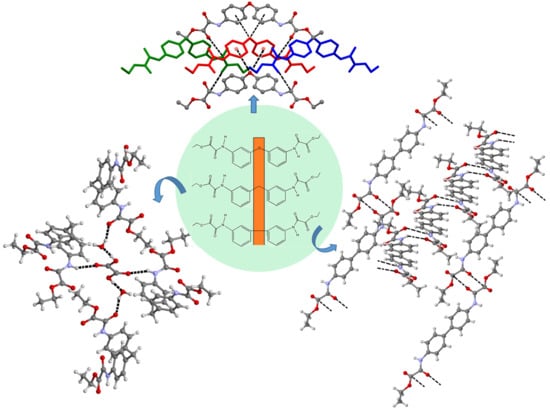
3.2. Supramolecular Architectures of 12•½(C2H2O4)•H2O and 2-3
3.3. DFT Calculations and Hirshfeld Surface Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ono, S.J.; Abelson, M.B. Allergic conjunctivitis: Update on pathophysiology and prospects for future treatment. J. Allergy Clin. Immunol. 2005, 115, 118–122. [Google Scholar] [CrossRef]
- La Rosa, M.; Lionetti, E.; Reibaldi, M.; Russo, A.; Longo, A.; Leonardi, S.; Tomarchio, S.; Avitabile, T.; Reibaldi, A. Allergic conjunctivitis: A comprehensive review of the literature. Italian J. Pediatr. 2013, 39, 18. [Google Scholar] [CrossRef] [Green Version]
- Das, D.; Khan, M.; Gul, A.; Alam, R. Safety and efficacy of Lodoxamide in vernal keratoconjunctivitis. J. Pak. Med. Assoc. 2011, 61, 239–241. [Google Scholar]
- Padilla-Martínez, I.I.; Chaparro-Huerta, M.; Martínez-Martínez, F.J.; Höpfl, H.; García-Báez, E.V. Diethyl N,N’-m-phenyl–enedioxamate. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, o825–o827. [Google Scholar] [CrossRef]
- González-González, J.S.; Martínez-Martínez, F.J.; Peraza-Campos, A.L.; Rosales-Hoz, M.J.; García-Báez, E.V.; Padilla-Martínez, I.I. Supramolecular architectures of conformationally controlled 1,3-phenyl-dioxalamic molecular clefts through hydrogen bonding and steric restraints. CrystEngComm 2011, 13, 4748–4761. [Google Scholar] [CrossRef] [Green Version]
- González-González, J.S.; Padilla-Martínez, I.I.; García-Báez, E.V.; Franco-Hernández, O.; Martínez-Martínez, F.J. Helical supramolecular assembly of N2,N2′-bis[3-(morpholin-4-yl)propyl]-N1,N1′-(1,2-phenylene)dioxalamide dimethyl sulfoxide monosolvate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2013, 69, 66–69. [Google Scholar] [CrossRef]
- González-González, J.S.; Martínez-Martínez, F.J.; García-Báez, E.V.; Cruz, A.; Morín-Sánchez, L.M.; Rojas-Lima, S.; Padilla-Martínez, I.I. Molecular Complexes of diethyl n,n′-1,3-phenyldioxalamate and resorcinols: Conformational switching through intramolecular three-centered hydrogen-bonding. Cryst. Growth Des. 2014, 14, 628–642. [Google Scholar] [CrossRef]
- González-González, J.S.; Zúñiga-Lemus, O.; Martínez-Martínez, F.J.; González, J.; García-Báez, E.V.; Padilla-Martínez, I.I. Mechanochemical complexation of diethyl N,N´-[1,3-(2-methyl)phenyl]dioxalamate and resorcinol: Conformational twist and x-ray helical supramolecular architecture. J. Chem. Crystallogr. 2015, 45, 244–250. [Google Scholar] [CrossRef]
- Ramírez-Milanés, E.G.; Martínez-Martínez, F.J.; Magaña-Vergara, N.E.; Rojas-Lima, S.; Avendaño-Jiménez, Y.A.; García-Báez, E.V.; Morín-Sánchez, L.M.; Padilla-Martínez, I.I. Positional isomerism and steric effects in the self-assemblies of phenylene bis-monothiooxalamides. Cryst. Growth Des. 2017, 17, 2513–2528. [Google Scholar] [CrossRef]
- Blay, G.; Fernández, I.; Pedro, J.R.; Ruiz-García, R.; Muñoz, M.C.; Cano, J.; Carrasco, R. A Hydrogen-bonded supramolecular meso-helix. Eur. J. Org. Chem. 2003, 2003, 1627–1630. [Google Scholar] [CrossRef]
- Martín, S.; Beitia, J.I.; Ugalde, M.; Vitoria, P.; Cortes, R. Diethyl N,N´-o-phenylenedioxamate. Acta Crystallogr. Sect. E Struct. Rep. Online 2002, 58, o913–o915. [Google Scholar] [CrossRef]
- Yang, W.; Liu, X. Diethyl N,N′-(p-phenylene)dioxamate. Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, o1852. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, W.X.C.; Pinheiro, C.B.; da Costa, M.M.; Fontes, A.P.S.; Nunes, W.C.; Lloret, F.; Julve, M.; Pereira, C.L.M. Crystal engineering applied to modulate the structure and magnetic properties of oxamate complexes containing the [Cu(bpca)]+ Cation. Cryst. Growth Des. 2016, 16, 4094–4107. [Google Scholar] [CrossRef]
- Lisnard, L.; Chamoreau, L.-M.; Li, Y.; Journaux, Y. Solvothermal synthesis of oxamate-based helicate: Temperature dependence of the hydrogen bond structuring in the solid. Cryst. Growth Des. 2012, 12, 4955–4962. [Google Scholar] [CrossRef] [Green Version]
- Dul, M.C.; Pardo, E.; Lescouëzec, R.; Journaux, Y.; Ferrando-Soria, J.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F.; Cangussue, D.; et al. Supramolecular coordination chemistry of aromatic polyoxalamide ligands: A metallosupramolecular approach toward functional magnetic materials. Coord. Chem. Rev. 2010, 254, 2281–2296. [Google Scholar] [CrossRef] [Green Version]
- Miljanic, S.; Frkanec, L.; Meic, Z.; Zinic, M. Gelation ability of novel oxamide-based derivatives bearing a stilbene as a photo-responsive unit. Eur. J. Chem. 2006, 2006, 1323–1334. [Google Scholar] [CrossRef]
- Chong-Canto, S.; García-Báez, E.V.; Martínez-Martínez, F.J.; Ramos-Organillo, A.A.; Padilla-Martínez, I.I. Mechanochemical synthesis and structure of the tetrahydrate and mesoporous anhydrous metforminium(2+)-N,N´-1,4-phenylenedioxalamic acid (1:2) salt: The role of hydrogen bonding and n-p* charge assisted interactions. Pharmaceutics 2020, 12, 998. [Google Scholar] [CrossRef]
- Basu, A.; Anasua Sarkar, A.; Basak, P. Immunoinformatics study of procyanidins as mast cell stabilizers. Pharmacogn. J. 2018, 10, 814–817. [Google Scholar] [CrossRef]
- Eggel, A.; Baravalle, G.; Hobi, G.; Kim, B.; Buschor, P.; Forrer, P.; Shin, J.S.; Vogel, M.; Stadler, B.M.; Dahinden, C.A.; et al. Accelerated dissociation of IgE-FcεRI complexes by disruptive inhibitors actively desensitizes allergic effector cells. J. Allergy Clin. Immunol. 2014, 133, 1709–1719. [Google Scholar] [CrossRef] [Green Version]
- Sellstedt, J.H.; Guinosso, C.J.; Begany, A.J.; Bell, S.C.; Rosenthale, M. Oxanilic acids, a new series of orally active antiallergic agents. J. Med. Chem. 1975, 18, 926–933. [Google Scholar] [CrossRef]
- Hall, C.M.; Wright, J.B. Anti-Allergic Oxanilate Compounds. U.S. Patent 4,061,791, 6 December 1977. [Google Scholar]
- McDonald, R. Reaction of oxalyl chloride with amine hydrochlorides. J. Org. Chem. 1959, 24, 1580–1581. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Bruker, A.X.S. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2008. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0-new features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Holzmann, N.; Stasch, A.; Jones, C.; Frenking, G. Structures and stabilities of group 13 adducts [(NHC)(EX3)] and [(NHC) 2 (E2Xn)](E= B to In; X= H, Cl; n= 4, 2, 0; NHC= N-Heterocyclic Carbene) and the search for hydrogen storage systems: A theoretical study. Chem. Eur. J. 2011, 17, 13517–13525. [Google Scholar] [CrossRef]
- Lin, Y.S.; Li, G.D.; Mao, S.P.; Chai, J.D. Long-range corrected hybrid density functionals with improved dispersion corrections. J. Chem. Theory Comput. 2013, 9, 263–272. [Google Scholar] [CrossRef]
- Baldridge, K.; Klamt, A. First principles implementation of solvent effects without outlying charge error. J. Chem. Phys. 1997, 106, 6622–6633. [Google Scholar] [CrossRef]
- Takano, Y.; Houk, K.N. Benchmarking the conductor-like polarizable continuum model (CPCM) for aqueous solvation free energies of neutral and ionic organic molecules. J. Chem. Theory Comput. 2005, 1, 70–77. [Google Scholar] [CrossRef]
- Zhurko, G.A.; Zhurko, D.A. Chemcraft Program, Academic Version 1.8. 2009. Available online: https://www.chemcraftprog.com (accessed on 18 November 2020).
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer 3.1; The University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Koga, T.; Kanayama, K.; Thakkar, A.J. Noninteger principal quantum numbers increase the efficiency of Slater-type basis sets. Chem. Phys. Lett. 1997, 62, 1–11. [Google Scholar] [CrossRef]
- Rodríguez, O.A.R.; Vergara, N.E.M.; Sánchez, J.P.M.; Martínez, M.T.S.; Sandoval, Z.G.; Cruz, A.; Organillo, A.R. Synthesis, crystal structure, antioxidant activity and dft study of 2-aryl-2,3-dihydro-4H-[1,3]thiazino[3,2-a]benzimidazol-4-One. J. Mol. Struct. 2020, 1199, 127036. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Meghdadi, S.; Khavasi, H.R.; Nalchigar, S. N,N′-(Methyl–enedi-p-phenylene)bis(pyridine-2-carboxamide). Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, o5492–o5493. [Google Scholar] [CrossRef]
- Gardner, K.H.; Blackwell, J. Structure of dimethyl 4,4′-methylenebis(phenylcarbamate): A model for MDI units in polyurethane hard segments. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1980, 36, 1972–1975. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in Solid State. Angew. Chem. Int. Ed. Engl. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Desiraju, G.R. The C-H···O Hydrogen Bond: Structural Implications and Supramolecular Design. Acc. Chem. Res. 1996, 29, 441–449. [Google Scholar] [CrossRef]
- Nishio, M. CH/interactions hydrogen bonds in crystals. CrystEngComm 2004, 6, 130–158. [Google Scholar] [CrossRef]
- Allen, F.H.; Baalham, C.A.; Lommerse, J.P.M.; Raithby, P.R. Carbonyl-Carbonyl Interactions can be Competitive with Hydrogen Bonds. Acta Crystallogr. Sect. B Struct. Sci. 1998, B54, 320–329. [Google Scholar] [CrossRef]
- Wang, W.Z.; Fan, N.T. Synthesis and Crystal Structure of 4,4′-Oxobis(propham). Acta Phys. Chim. Sin. 2003, 19, 75–78. [Google Scholar] [CrossRef]











| Parameter | 12•½(C2H2O4)•H2O | 2 | 3 |
|---|---|---|---|
| CCDC | 795132 | 795130 | 795134 |
| Chemical formula | 2(C21H22N2O6)•0.5(C2H2O4)•H2O | C20H20N2O7 | 2(C20H20N2O6) |
| Mr molecular weight | 859.84 | 400.38 | 768.77 |
| Crystal system, space group | Triclinic, P-1 | Orthorhombic, Pbnb | Triclinic, P-1 |
| Temperature (K) | 173 | 173 | 293 |
| a, b, c (Å) | 10.455 (2), 13.174 (3), 16.443 (3) | 7.9088 (2), 8.5779 (3), 28.4375 (9) | 8.5431 (11), 10.7978 (13), 16.105 (2) |
| α, β, γ (°) | 87.97 (3), 81.08 (3), 70.51 (3) | 90, 90, 90 | 108.892 (2), 93.354 (2), 90.916 (2) |
| V (Å3) | 2108.9 (8) | 1929.23 (10) | 1402.3 (3) |
| Z | 2 | 4 | 3 |
| Crystal size (mm) | 0.40 × 0.30 × 0.25 | 0.50 × 0.35 × 0.21 | 0.48 × 0.44 × 0.39 |
| Tmin, Tmax | 0.861, 0.862 | 0.861, 0.862 | 0.861, 0.862 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 20,384, 7437, 6380 | 5598, 1973, 1380 | 13,452, 4922, 4303 |
| Rint | 0.026 | 0.033 | 0.029 |
| (sin θ/λ)max (Å−1) | 0.595 | 0.65 | 0.595 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.051, 0.123, 1.12 | 0.056, 0.141, 1.06 | 0.064, 0.167, 1.13 |
| No. of reflections | 7437 | 1973 | 4922 |
| No. of parameters | 591 | 160 | 379 |
| No. of restraints | 4 | 6 | 0 |
| Δρmax, Δρmin (e Å−3) | 0.18, −0.20 | 0.13, −0.12 | 0.25, −0.25 |
| Comp. | D―H∙∙∙A | D―H/Å | H···A/Å | D···A/Å | D―H···A/° |
|---|---|---|---|---|---|
| 12•½(C2H2O4)•H2O | N7-H7∙∙∙O60 i | 0.88 | 2.16 | 2.980(2) | 154 |
| N20-H20∙∙∙O34 ii | 0.88 | 2.24 | 3.045(2) | 151 | |
| N32-H32∙∙∙O22 ii | 0.88 | 2.37 | 3.153(3) | 148 | |
| N45-H45∙∙∙O8 i | 0.88 | 2.22 | 3.063(2) | 159 | |
| N45-H45∙∙∙O10 i | 0.88 | 2.45 | 3.037(2) | 125 | |
| O61-H61∙∙∙O70 | 0.84 | 1.73 | 2.569(2) | 178 | |
| O70-H70A∙∙∙O8 | 0.86 | 2.02 | 2.864(2) | 167 | |
| O70-H70B∙∙∙O46 iii | 0.82 | 2.03 | 2.819(2) | 159 | |
| O70-H70B∙∙∙O48 iii | 0.82 | 2.54 | 3.141(2) | 131 | |
| C44-H44∙∙∙O8 i | 0.95 | 2.56 | 3.367(3) | 143 | |
| C18-H18∙∙∙Cg(1) v | 0.95 | 3.018 | 3.848 | 147 | |
| 2 | N7-H7∙∙∙O8 iv | 0.86 | 2.29 | 3.082(2) | 154 |
| C3-H3∙∙∙Cg(2) ix | 0.93 | 3.268 | 4.104 | 151 | |
| 3 | N7-H7∙∙∙O48 v | 0.86 | 2.20 | 3.019(3) | 158 |
| N27-H27∙∙∙O49 vi | 0.86 | 2.50 | 3.232(3) | 143 | |
| C2-H2∙∙∙O48 viii | 0.93 | 2.44 | 3.210(3) | 140 | |
| C22-H22∙∙∙O49 vi | 0.93 | 2.54 | 3.374(3) | 149 | |
| C12-H12C ∙∙∙Cg(2) x | 0.98 | 3.285 | 9.979 | 131 | |
| C32-H32B∙∙∙Cg(2) xi | 0.96 | 3.213 | 4.012 | 142 |
| Compound | In Gas Phase (kcal/mol) | |
|---|---|---|
| syn | anti | |
| 1 | 5.611 | 0 |
| 2 | 5.617 | 0 |
| 3 | 5.505 | 0 |
| Molecule/Interaction | Gas Phase (Å) | |
|---|---|---|
| syn | anti | |
| 1 | ||
| N7-H7···O9 | 2.060 | 2.104 |
| 2 | ||
| N7-H7···O9 | 2.059 | 2.102 |
| 3 | ||
| N7-H7···O9 | 2.059 | 2.103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-González, J.S.; Magaña-Vergara, N.E.; García-Báez, E.V.; Padilla-Martínez, I.I.; Mojica-Sánchez, J.P.; Martínez-Martínez, F.J. Crystal Structure and Supramolecular Architecture of Antiallergic Diphenylene Diethyl Dioxalamates. Crystals 2020, 10, 1048. https://doi.org/10.3390/cryst10111048
González-González JS, Magaña-Vergara NE, García-Báez EV, Padilla-Martínez II, Mojica-Sánchez JP, Martínez-Martínez FJ. Crystal Structure and Supramolecular Architecture of Antiallergic Diphenylene Diethyl Dioxalamates. Crystals. 2020; 10(11):1048. https://doi.org/10.3390/cryst10111048
Chicago/Turabian StyleGonzález-González, Juan Saulo, Nancy Evelyn Magaña-Vergara, Efrén Venancio García-Báez, Itzia Irene Padilla-Martínez, Juan Pablo Mojica-Sánchez, and Francisco Javier Martínez-Martínez. 2020. "Crystal Structure and Supramolecular Architecture of Antiallergic Diphenylene Diethyl Dioxalamates" Crystals 10, no. 11: 1048. https://doi.org/10.3390/cryst10111048
APA StyleGonzález-González, J. S., Magaña-Vergara, N. E., García-Báez, E. V., Padilla-Martínez, I. I., Mojica-Sánchez, J. P., & Martínez-Martínez, F. J. (2020). Crystal Structure and Supramolecular Architecture of Antiallergic Diphenylene Diethyl Dioxalamates. Crystals, 10(11), 1048. https://doi.org/10.3390/cryst10111048