Heterometallic Complexes Containing the NiII-LnIII-NiII Moiety—Structures and Magnetic Properties
Abstract
:1. Introduction
2. Discussion
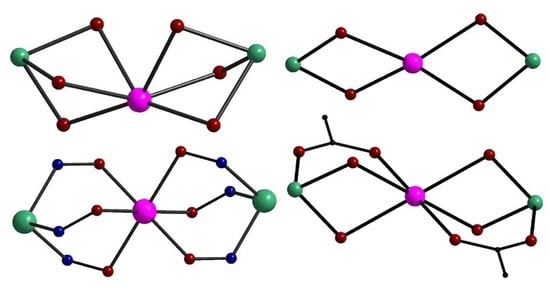
2.1. Trinuclear NiII-LnIII-NiII Complexes
2.1.1. Tripodal Polydentate Schiff Base Ligands and Reduced Schiff Base Ligands
2.1.2. Other Schiff Base Ligands
2.1.3. Miscellaneous Ligands
2.2. Oligonuclear Complexes Containing the NiII-LnIII-NiII unit
2.3. Coordination Polymers Containing NiII-LnIII-NiII Subunits
2.3.1. 1D Coordination Polymers
2.3.2. 2D Coordination Polymers
2.3.3. 3D Coordination Polymers
3. Summary
Supplementary Materials
Funding
Conflicts of Interest
References
- Lis, T. Preparation, structure, and magnetic properties of dodecanuclear mixed-valence manganese carboxylate. Acta Cryst. 1980, 36, 2042–2046. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Sessoli, R. Alternating current susceptibility, high field magnetization, and millimeter band EPR evidence for a ground S = 10 state in [Mn12O12(CH3COO)16(H2O)4].2CH3COOH.4H2O. J. Am. Chem. Soc. 1991, 113, 5873–5874. [Google Scholar] [CrossRef]
- Sessoli, R.; Tsai, H.-L.; Schake, A.R.; Wang, S.; Vincent, J.B.; Folting, K.; Gatteschi, D.; Christou, G.; Hendrickson, D.N. High-spin molecules: [Mn12O12(O2CR)16(H2O)4]. J. Am. Chem. Soc. 1993, 115, 1804–1816. [Google Scholar] [CrossRef]
- Weighardt, K.; Pohl, D.; Jibril, I.; Huttner, G. Hydrolysis products of the monomeric amine complex (C6H15N3)FeCl3: The structure of the octameric iron(III) cation of {[(C6H15N3)6Fe8(μ3-O)2(m2-OH)12]Br7(H2O)}Br.8H2O. Angew. Chem. Int. Ed. Engl. 1984, 23, 77–78. [Google Scholar] [CrossRef]
- Delfs, C.; Gatteschi, D.; Pardi, L.; Sessoli, R.; Weighardt, K.; Hank, D. Magnetic properties of an octanuclear iron(III) cation. Inorg. Chem. 1993, 32, 3099–3103. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.-Y.; Kaizu, Y. Lanthanide double-decker complexes functioning as magnets at the single-molecule level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef]
- Zadrozny, J.M.; Xiao, D.J.; Atanasov, M.; Long, G.J.; Grandjean, F.; Neese, F.; Long, J.R. Magnetic blocking in a linear iron(I) complex. Nat. Chem. 2013, 5, 577–581. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Lalioti, N.; Sangregorio, C.; Sessoli, R.; Venturi, G.; Vindigni, A.; Rettori, A.; Pini, M.G.; Novak, M.A. Cobalt(II)-nitronyl nitroxide chains as molecular magnetic nanowires. Angew. Chem. Int. Ed. Engl. 2001, 40, 1760–1763. [Google Scholar] [CrossRef]
- Lada, Z.G.; Katsoulakou, E.; Perlepes, S.P. Synthesis and Chemistry of Single-Molecule Magnets. In Single-Molecule Magnets-Molecular Architectures and Building Blocks for Spintronics; Holynska, M., Ed.; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2019. [Google Scholar]
- Polyzou, C.D.; Efthymiou, C.G.; Escuer, A.; Cuhna-Silva, L.; Papatriantafyllopoulou, C.; Perlepes, S.P. In search of 3d/4f-metal single-molecule magnets: Nickel(II)/lanthanide(III) coordination clusters. Pure Appl. Chem. 2013, 85, 315–327. [Google Scholar] [CrossRef]
- Papatriantafyllopoulou, C.; Estrader, M.; Efthymiou, C.G.; Dermitzaki, D.; Gkotsis, K.; Terzis, A.; Diaz, C.; Perlepes, S.P. In search for mixed transition metal/lanthanide single-molecule magnets: Synthetic routes to NiII/TbIII and NiII/DyIII clusters featuring a 2-pyridyl oximate ligand. Polyhedron 2009, 28, 1652–1655. [Google Scholar] [CrossRef]
- Osa, S.; Kido, T.; Matsumoto, N.; Re, N.; Pochaba, A.; Mrozinski, J. A tetranuclear 3d-4f single molecule magnet: [CuIILTbIII(hfac)2]2. J. Am. Chem. Soc. 2004, 126, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Benchini, A.; Benelli, C.; Caneschi, A.; Carlin, R.L.; Dei, A.; Gatteschi, D. Crystal and molecular structure of and magnetic coupling in two complexes containing gadolinium(III) and copper(II) ions. J. Am. Chem. Soc. 1985, 107, 8128–8136. [Google Scholar] [CrossRef]
- Costes, J.-P.; Dahan, F.; Dupuis, A.; Laurent, J.P. A genuine example of a discrete bimetallic (Cu, Gd) complex: Structural determination and magnetic properties. Inorg. Chem. 1996, 35, 2400–2402. [Google Scholar] [CrossRef] [PubMed]
- Pasatoiu, T.D.; Sutter, J.-P.; Madalan, A.M.; Fellah, F.Z.C.; Duhayon, C.; Andruh, M. Preparation, crystal structures, and magnetic features for a series of dinuclear [NiIILnIII] Schiff-base complexes: Evidence for slow relaxation of the magnetization for the DyIII derivative. Inorg. Chem. 2011, 50, 5890–5898. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Costes, J.-P.; Kishima, Y.; Kojima, M.; Sunatsuki, Y.; Bréfuel, N.; Tuchagues, J.-P.; Vendier, L.; Wernsdorfer, W. Face-sharing heterotrinuclear MII-LnIII-MII (M = Mn, Fe, Co, Zn; Ln = La, Gd, Tb, Dy) complexes: Synthesis, structures, and magnetic properties. Inorg. Chem. 2010, 49, 9125–9135. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, V.; Pandian, B.M.; Boomishankar, R.; Steiner, A.; Vittal, J.J.; Houri, A.; Clérac, R. Trinuclear heterobimetallic Ni2Ln complexes [L2Ni2Ln](ClO4) (Ln = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, and Er; LH3 = (S)P[N(Me)N=CH-C6H3-2-OH_3-OMe]3): From simple paramagnetic complexes to single-molecule magnet behavior. Inorg. Chem. 2008, 47, 4918–4929. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Rajeshkumar, T.; Chandrasekhar, V.; Rajaraman, G. Theoretical studies on {3d-Gd} and {3d-Gd-3d} complexes: Effect of metal substitution on the effective exchange interaction. Polyhedron 2013, 66, 81–86. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sunatsuki, Y.; Kojima, M.; Akashi, H.; Tsuchimoto, M.; Re, N.; Osa, S.; Matsumoto, N. Ferromagnetic NiII-GdIII interactions in complexes with NiGd, NiGdNi, and NiGdGdNi cores supported by tripodal ligands. Chem. Commun. 2004, 1048–1049. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sunatsuki, Y.; Ishida, H.; Kajima, M.; Akashi, H.; Re, N.; Matsumoto, N.; Pochaba, A.; Mroziński, J. Synthesis, structures, and magnetic properties of double face-sharing heterotrinuclear NiII-LnIII-NiII (Ln = Eu, Gd, Tb, and Dy) complexes. Bull. Chem. Soc. Jpn. 2008, 81, 598–605. [Google Scholar] [CrossRef]
- Costes, J.-P.; Yamaguchi, T.; Kojima, M.; Vendier, L. Experimental evidence for the participation of 5d GdIII orbitals in the magnetic interaction in Ni-Gd complexes. Inorg. Chem. 2009, 48, 5555–5561. [Google Scholar] [CrossRef]
- Yao, M.-X.; Zhu, Z.-X.; Lu, X.-Y.; Deng, X.-W.; Jing, S. Rare single-molecule magnets with six-coordinate LnIII ions exhibiting a trigonal antiprism configuration. Dalton Trans. 2016, 45, 10689–10695. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Read, P.W.; Hibbs, D.E.; Hursthouse, M.B.; Abdul Malik, K.M.; Patrick, B.O.; Rettig, S.J.; Seid, M.; Summers, D.A.; Pink, M.; et al. Coaggregation of paramagnetic d- and f-block metal ions with a podand-framework amine phenol ligand. Inorg. Chem. 2000, 39, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Bayly, S.R.; Xu, Z.; Patrick, B.O.; Rettig, S.J.; Pink, M.; Thompson, R.C.; Orvig, C. d/f complexes with uniform coordination geometry: Structural and magnetic properties of an LnNi2 core supported by a heptadentate amine phenol ligand. Inorg. Chem. 2003, 42, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, A.; Reglinski, J.; Kennedy, A.R. The use of hydrogenated Schiff base ligands in the synthesis of milti-metallic compounds. Inorg. Chim. Acta 2009, 362, 1267–1274. [Google Scholar] [CrossRef]
- Wen, H.-R.; Dong, P.-P.; Liu, S.-J.; Liao, J.-S.; Liang, F.-Y.; Liu, C.-M. 3d-4f heterometallic trinuclrear complexes derived from amine-phenol tripodal ligands exhibiting magnetic and luminescent properties. Dalton Trans. 2017, 46, 1153–1162. [Google Scholar] [CrossRef]
- Barta, C.A.; Bayly, S.R.; Read, P.W.; Patrick, B.O.; Thompson, R.C.; Orvig, C. Molecular architectures for trimetallic d/f/d complexes: Structural and magnetic properties of a LnNi2 core. Inorg. Chem. 2008, 47, 2280–2293. [Google Scholar] [CrossRef]
- Comba, P.; Enders, M.; Groẞhauser, M.; Hiller, M.; Müller, D.; Wadepohl, H. Solution and solid state structures and magnetism of a series of linear trinuclear compounds with a hexacoordinate LnIII and two terminal NiII centers. Dalton Trans. 2017, 46, 138–149. [Google Scholar] [CrossRef]
- Wen, H.-R.; Zhang, J.-L.; Liang, F.-Y.; Yang, K.; Liu, S.-J.; Liao, J.-S.; Liu, C.-M. TbIII/3d-TbIII clusters derived from a 1,4,7-triazacyclononane-based hexadentate ligand with field-induced slow magnetic relaxation and oxygen-sensitive luminescence. New J. Chem. 2019, 43, 4067–4074. [Google Scholar] [CrossRef]
- Upadhyay, A.; Komatireddy, N.; Ghirri, A.; Tuna, F.; Langley, S.K.; Srivastava, A.K.; Sañudo, E.C.; Moubaraki, B.; Murray, K.S.; McInnes, E.J.L.; et al. Synthesis and magnetothermal properties of a ferromagnetically coupled NiII-GdIII-NiII cluster. Dalton Trans. 2014, 43, 259–266. [Google Scholar] [CrossRef]
- Upadhyay, A.; Das, C.; Langley, S.K.; Murray, K.S.; Srivastava, A.K.; Shanmugam, M. Heteronuclear Ni(II)-Ln(III) (Ln = La, Pr, Tb, Dy) complexes: Synthesis and single-molecule magnet behavior. Dalton Trans. 2016, 45, 3616–3626. [Google Scholar] [CrossRef]
- Ahmed, N.; Das, C.; Vaidya, S.; Kumar Srivastava, A.; Langley, S.K.; Murray, K.S.; Shanmugam, M. Probing the magnetic and magnetothermal properties of M(II)-Ln(III) complexes (where M(II) = Ni or Zn; Ln(III) = La or Pr or Gd). Dalton Trans. 2014, 43, 17375–17384. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dey, A.; Kundu, S.; Biswas, S.; Mota, A.J.; Colacio, E.; Chandrasekhar, V. Linear {NiII-LnIII-NiII} complexes containing twisted planar Ni(μ-phenolate)2Ln fragments: Synthesis, structure, and magnetothermal properties. Chem. Asian. J. 2014, 9, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulou, A.N.; Pissas, M.; Psycharis, V.; Sanakis, Y.; Raptopoulou, C.P. Trinuclear NiII-LnIII-NiII complexes with Schiff base ligands: Synthesis, structure, and magnetic properties. Molecules 2020, 25, 2280. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Hu, R.-H.; Liu, D.-S.; Wu, Q. Adjustment of the structures and biological activities by the ratio of NiL to RE for two sets of Schiff base complexes [(NiL)nRE] (n = 1 or 2; RE = La or Ce). Inorg. Chem. Comm. 2011, 14, 396–398. [Google Scholar] [CrossRef]
- Ali Güngör, S.; Kose, M. Synthesis, crystal structure, photoluminescence and electrochemical properties of a sandwiched Ni2Ce complex. J. Mol. Struct. 2017, 1150, 274–278. [Google Scholar] [CrossRef]
- Cristovao, B.; Kłak, J.; Miroslaw, B. Synthesis, crystal structures and magnetic behavior of NiII-4f-NiII compounds. Polyhedron 2012, 43, 47–54. [Google Scholar] [CrossRef]
- Cristóvão, B.; Kłak, J.; Pełka, R.; Miroslaw, B.; Hnatejko, Z. Heterometallic trinuclear 3d-4f-3d compounds based on a hexadentate Schiff base ligand. Polyhedron 2014, 68, 180–190. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, A. Coordination of metalloligand [NiL] (H2L = salen type N2O2 Schiff base ligand) to the f-block elements: Structural elucidation and spectrophotometric investigation. Inorg. Chim. Acta 2016, 442, 64–69. [Google Scholar] [CrossRef]
- Costes, J.-P.; Donnadieu, B.; Gheorghe, R.; Novitchi, G.; Tuchagues, J.-P.; Vendier, L. Di- or trinuclear 3d-4f Schiff base complexes: The role of anions. Eur. J. Inorg. Chem. 2008, 5235–5244. [Google Scholar] [CrossRef]
- Bhunia, A.; Yadav, M.; Lan, Y.; Powell, A.K.; Menges, F.; Riehn, C.; Niedner-Schatteburg, G.; Jana, P.P.; Riedel, R.; Harms, K.; et al. Trinuclear nickel-lanthanide compounds. Dalron Trans. 2013, 42, 2445–2450. [Google Scholar] [CrossRef]
- Deacon, G.B.; Forsyth, C.M.; Junk, P.C.; Leary, S.G. A rare earth alloy as a synthetic reagent: Contrasting homometallic rare earth and heterobimetallic outcomes. New J. Chem. 2006, 30, 592–596. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, F.; Song, Y.-M.; Feng, X.-F.; Luo, M.-B.; Liao, Z.-W.; Sun, G.-M.; Tian, X.-Z.; Yuan, Z.-J. The first one-pot synthesis of multinuclear 3d-4f metal-organic compounds involving a polytopic N,O-donor ligand formed in situ. Cryst. Growth Des. 2012, 12, 2158–2161. [Google Scholar] [CrossRef]
- Chesman, A.S.R.; Turner, D.R.; Moubaraki, B.; Murray, K.S.; Deacon, G.B.; Batten, S.R. Synthesis and magnetic properties of a series of 3d/4f/3d heterometallic trinuclear complexes incorporating in situ ligand formation. Inorg. Chim. Acta 2012, 389, 99–106. [Google Scholar] [CrossRef]
- Shiga, T.; Ito, N.; Hidaka, A.; Okawa, H.; Kitagawa, S.; Ohba, M. A series of trinuclear CuIILnIIICuII complexes derived from 2,6-di(acetoacetyl)pyridine: Synthesis, structure, and magnetism. Inorg. Chem. 2007, 46, 3492–3501. [Google Scholar] [CrossRef]
- Trieu, T.N.; Nguyen, M.H.; Abram, U.; Nguyen, H.H. Syntheses and structures of new trinuclear MIILnMII (M = Ni, Co; Ln = Gd, Ce) complexes with 2,6-bis(acetobenzoyl)pyridine. Z. Anorg. Allg. Chem. 2015, 641, 863–870. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Jegathesh, J.J.; Takiden, A.; Hauenstein, D.; Pham, C.T.; Le, C.D.; Abram, U. 2,6-dipicolinoylbis(N,N-dialkylthioureas) as versatile building blocks for oligo- and polynuclear architectures. Dalton Trans. 2016, 45, 10771–10779. [Google Scholar] [CrossRef] [Green Version]
- Burkovskaya, N.P.; Orlova, E.V.; Kiskin, M.A.; Efimov, N.N.; Bogomyakov, A.S.; Fedin, M.V.; Kolotilov, S.V.; Minin, V.V.; Aleksandrov, G.G.; Sidorov, A.A.; et al. Synhtesis, structure, and magnetic properties of heterometallic trinuclear complexes {MII-LnIII-MII} (MII = Ni, Cu; LnIII = La, Pr, Sm, Eu, Gd). Russ. Chem. Bull Int. Ed. 2011, 60, 2490–2503. [Google Scholar] [CrossRef]
- Kalogridis, C.; Palacios, M.A.; Rodríguez-Diéguez, A.; Mota, A.J.; Choquesillo-Lazarte, D.; Brechin, E.K.; Colacio, E. Heterometallic oximato-bridged linear trinuclear NiII-MIII-NiII (MIII = Mn, Fe, Tb) complexes constructed with the fac-O3 [Ni(HL)3]− metalloligand (H2L = pyrimidine-2-carboxamide oxime): A theoretical and experimental magneto-structural study. Eur. J. Inorg. Chem. 2011, 5225–5232. [Google Scholar] [CrossRef]
- Efthymiou, C.G.; Georgopoulou, A.N.; Papatriantafyllopoulou, C.; Terzis, A.; Raptopoulou, C.P.; Escuer, A.; Perlepes, S.P. Initial employment of di-2-pyridyl ketone as a route to nickel(II)/lanthanide(III) clusters: Triangular Ni2Ln complexes. Dalton Trans. 2010, 29, 8603–8605. [Google Scholar] [CrossRef]
- Georgopoulou, A.N.; Efthymiou, C.G.; Papatriantafyllopoulou, C.; Psycharis, V.; Raptopoulou, C.P.; Manos, M.; Tasiopoulos, A.J.; Escuer, A.; Perlepes, S.P. Triangular NiII2LnIII and NiII2YIII complexes derived from di-2-pyridyl ketone: Synthesis, structures and magnetic properties. Polyhedron 2011, 30, 2978–2986. [Google Scholar] [CrossRef]
- Gheorghe, R.; Andruh, M.; Costes, J.-P.; Donnadieu, B.; Schmidtmann, M.; Müller, A. Making 3d-4f hexanuclear clusters from heterotrinuclear cationic building blocks. Inorg. Chim. Acta 2007, 360, 4044–4050. [Google Scholar] [CrossRef]
- Dinca, A.S.; Shova, S.; Ion, A.E.; Maxim, C.; Lloret, F.; Julve, M.; Andruh, M. Ascorbic acid decomposition into oxalate ions: A simple synthetic route towards oxalate-bridged heterometallic 3d-4f clusters. Dalton Trans. 2015, 44, 7148–7151. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.-Q.; Jiang, X.; Wu, S.-Q.; Liu, C.-M.; Cui, A.-L.; Kou, H.-Z. Slow magnetization relaxation in NiIIDyIIIFeIII molecular cycles. Inorg. Chem. 2015, 54, 1206–1208. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-J.; Hu, K.-Q.; Liu, C.-M.; Cui, A.-L.; Kou, H.-Z. Metallocyclic Ni4Ln2M2 single-molecule magnets. Dalton Trans. 2017, 46, 6544–6552. [Google Scholar] [CrossRef]
- Dhers, S.; Costes, J.-P.; Guionneau, P.; Paulsen, C.; Vendier, L.; Sutter, J.-P. On the importance of ferromagnetic exchange between transition metals in field-free SMMs: Examples of ring-shaped hetero-trimetallic [(LnNi2){W(CN)8}]2 compounds. Chem. Commun. 2015, 51, 7875–7878. [Google Scholar] [CrossRef]
- Ghosh, S.; Mahapatra, P.; Kanetomo, T.; Drew, M.G.B.; Ishida, T.; Ghosh, A. Syntheses, crystal structure and magnetic properties of an unprecedented one-dimensional coordination polymer derived from an {(NiL)2Ln} node and a pyrazine spacer (H2L = N,N’-bis(salicylidene)-1,3-propanediamine, Ln = Gd, Tb and Dy). ChemistrySelect 2016, 1, 2722–2729. [Google Scholar] [CrossRef]
- Ion, A.E.; Nica, S.; Madalam, A.M.; Maxim, C.; Julve, M.; Lloret, F.; Andruh, M. One-dimensional coordination polymers constructed from di- and trinuclear {3d-4f} tectons. A new useful spacer in crystal engineering: 1,3-bis(4-pyridyl)azulene. CrystEngComm 2014, 16, 319–327. [Google Scholar] [CrossRef]
- Palacios, M.A.; Morlieras, J.; Herrera, J.M.; Mota, A.J.; Brechin, E.K.; Triki, S.; Colacio, E. Synthetic ability of dinuclear mesocates containing 1,3-bis(diazinecarboxamide)benzene bridging ligands to form complexes of increased nuclearity. Crystal structures, magnetic properties and theoretical studies. Dalton Trans. 2017, 46, 10469–10483. [Google Scholar] [CrossRef] [Green Version]
- Colacio, E.; Palacios, M.A.; Rodríguez-Diéguez, A.; Mota, A.J.; Herrera, J.M.; Choquesillo-Lazarte, D.; Clérac, R. 3d-3d-4f chain complexes constructed using the dinuclear metalacyclic complex [Ni2(mbpb)3]2− [H2mbpb = 1,3-bis(pyridine-2-carboxamide)benzene] as a ligand: Synthesis, structures, and magnetic properties. Inorg. Chem. 2010, 49, 1826–1833. [Google Scholar] [CrossRef]
- Wang, D.; Niu, C.-J.; Li, X.-Z.; Zhu, L.-N.; Hao, P.-P. Syntheses, crystal structures and properties of lanthanide complexes featuring infinite molecular rectangle strands constructed from a new macrocyclic metalloligand. Inorg. Chim. Acta 2012, 391, 20–27. [Google Scholar] [CrossRef]
- He, Z.; He, C.; Gao, E.-Q.; Wang, Z.-M.; Yang, X.-F.; Liao, C.-S.; Yan, C.-H. Lanthanide-transition heterometallic extended structures with novel orthogonal metalloligand as building block. Inorg. Chem. 2003, 42, 2206–2208. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-X.; Guo, W.-B.; Du, Z.-X.; Huang, W.-P. Monometallic nickel and bimetallic nickel-europium complexes based on oxydiacetic acid and N-heterocyclic ligands. Inorg. Chim. Acta 2011, 375, 290–297. [Google Scholar] [CrossRef]
- Liu, F.-C.; Zeng, Y.-F.; Jiao, J.; Li, J.-R.; Bu, X.-H.; Ribas, J.; Batten, S.R. Novel heterometallic 3d-4f metal-azido complex of mixed ligands with unprecedented structure type: Synthesis, structure, and magnetic properties. Inorg. Chem. 2006, 45, 6129–6131. [Google Scholar] [CrossRef] [PubMed]
- Mondal, K.C.; Sengupta, O.; Dutta, P.; Seehra, M.; Nayak, S.K.; Mukherjee, P.S. Three-dimensional 3d-4f heterometallic polymers containing both azide and carboxylate as co-ligands. Inorg. Chim. Acta 2009, 362, 1913–1917. [Google Scholar] [CrossRef]


























| Complex | NiII Coordination Mode | LnIII Coordination Mode | Ni-Ln-Ni Angle (o) | LnIII | Ref. |
|---|---|---|---|---|---|
| [(NiL1)2Ln](ClO4) | N3O3 | O12 | 176.2–178.7 | La-Er except Pm | [17,18] |
| [(NiL2)2Ln](NO3) | N3O3 | O8 | 139.9–143.8 | Eu, Gd, Tb, Dy | [19,20] |
| [(NiL3)2Ln](NO3) | N3O3 | O12 | 178.4–178.7 | Gd | [21] |
| [(NiL4)2Ln](NO3) | N3O3 | O6/O7 | 177.1–177.8 | Gd, Tb, Dy | [22] |
| [(NiL4)2Ln](ClO4) | N3O3 | O6 | 174.6 | Dy | [22] |
| [(NiL5)2Ln(solv)x](ClO4) a | N3O3 | O6/O7/O8 | 142.5–157.7 | La, Dy, Yb | [23] |
| [(NiL6)2Ln(MeOH)](NO3) | N4O2 | O7 | 139.6–143.9 | La, Sm, Tb, Er, Lu | [24,25] |
| [(NiL6)2Ln(MeOH)](ClO4) | N4O2 | O7 | 143.9 | Pr | [24] |
| [(NiL7)2Ln](ClO4)2 | N4O2 | O8 | 113.0, 113.3 | Gd, Dy | [26] |
| [(NiL8)2Ln(MeCN)2](ClO4) | N3O3 | N2O6 | 142.4 | Gd | [27] |
| [(NiL8)2Ln](ClO4) | N3O3 | O6 | 176.4 | Yb | [27] |
| [(NiL9)2Ln(solv)x](ClO4) b | N3O3 | O6, O7 | 165.1–178.8 | Y, Ce-Lu except Pm, Yb | [28] |
| [(NiL10)2Ln](ClO4) | N3O3 | O6 | 180.0 | Tb | [29] |
| [(NiL11)2Ln](NO3) | N3O3 | O12 | 179.7–179.9 | La, Pr, Gd, Tb | [30,31] |
| [{Ni(HL12)2}2Ln(NO3)](NO3)2 | N2O4 | O10 | 169.2 | La | [32] |
| [{Ni(H3L13)2}2Ln](NO3)3 | N2O4 | O8 | 179.3–179.6 | Gd, Tb, Dy, Ho | [33] |
| [{Ni(H3L14)2}2Ln(O2CMe)2](NO3)3 | N2O4 | O8 | 180.0 | Gd, Tb | [34] |
| [(NiL15)2Ln(NO3)2](NO3) | N2O2 | O12 | 67.8, 67.9 | La, Ce | [35] |
| [(NiL16)2Ln(NO3)2](NO3) | N2O2 | O12 | 62.1 | Ce | [36] |
| [(NiL17)2Ln(O2CMe)2(MeOH)2](NO3) | N2O4 | O10 | 180.0 | La, Nd, Ce, Pr | [37,38] |
| [(NiL18)2Ln(NO3)3] | N2O2 | O10 | 122.5 | Ce | [39] |
| [{Ni(L19)(H2O)}2Ln(H2O)](trif)3 c | N2O3 | O9 | 179.0, 177.2 | Gd, Eu | [21,40] |
| [{Ni(H2L20)2(tren)2}2Ln](NO3)3 d | N5O | O8 | 93.0–95.3 | Gd, Dy, Er, Lu | [41] |
| [{Ni(L21)3}2Ln(L21)] | N3O3 | NO7 | 129.6 | La | [42] |
| [{Ni(L22)1.5}2Ln(OH)] | N3O3 | O9 | 180.0 | Eu, Gd, Tb | [43] |
| (Me4N)[{Ni(L23)3}2Ln(L23)2] | N6 | N2O8 | 141.3–142.5 | La, Ce, Pr, Nd, Sm | [44] |
| (Et4N)2[{Ni(L23)3}2Ln(dcnm)2](ClO4)2 e | N6 | N2O8 | 133.1, 133.2 | La, Ce | [44] |
| [(NiL24)2Ln(NO3)2(MeOH)4](NO3) | O6 | N2O8 | 180.0 | La, Ce, Pr, Nd, Sm, Eu | [45] |
| [(NiL24)2Ln(NO3)2(H2O)2(MeOH)2](NO3) | O6 | N2O8 | 177.6–177.8 | Sm, Eu, Gd | [45] |
| [(NiL24)2Ln(NO3)3(MeOH)4] | O6 | N2O8 | 172.6–172.9 | Gd, Tb, Dy | [45] |
| [(NiL24)2Ln(NO3)2(H2O)(MeOH)3](NO3) | O6 | N2O8 | 179.4–179.5 | Ho, Er, Tm, Yb, Lu | [45] |
| [(NiL25)2Ln(O2CMe)3(MeOH)x] f | O6 | N2O7,N2O8 | 178.0–178.2 | Ce, Gd | [46] |
| [(NiL25)2Ln(O2CPh)3(solv)x] g | O6 | N2O8 | 176.6 | Gd | [46] |
| [(NiL26)2Ln(O2CMe)3(MeOH)2] | O4S2 | N2O8 | 176.9 | Pr | [47] |
| [{Ni(piv)3(bpy)}2Ln(NO3)] h | N2O4 | O8 | 152.9, 153.3 | Sm, Gd | [48] |
| [{Ni(piv)3(Hpiv)(MeCN)}2Ln(NO3)] | NO5 | O8 | 144.0 | Sm | [48] |
| [{Ni(L27)3}2Ln](NO3) | N3O3 | O6 | 180.0 | Tb | [10,11] |
| [(Ni(HL28)3}2Ln](NO3) | N3O3 | O6 | 180.0 | Tb | [49] |
| [Ni2(L29’)3(L29’’)Ln(NO3)(H2O)](ClO4)2 | N3O3 | N2O6 | 54.2, 54.4 | Gd, Tb | [50,51] |
| [Ni2(L29’)4Ln(NO3)(H2O)][Ln(NO3)5](ClO4)2 | N3O3 | N2O6 | 53.7 | Tb, Dy, Y | [50,51] |
| [{{(NiL30(H2O))(N(CN)2)}2Ln}2(μ-NO3)](OH) | N3O3 | O9, O10 | 175.7 | Pr | [52] |
| [{Ni2(L24)Ln(H2O)4}2(C2O4)3] | O6 | N2O8 | 178.6 | Gd | [53] |
| [{Ni(L19)2}2Ln(H2O)Fe(CN)6]2 | N3O2 | O9 | 179.1 | Dy | [54] |
| [(Ni(L19)2}2Ln(H2O)Cr(CN)6]2 | N3O2 | O9 | 177.6–178.5 | Y, Gd, Tb, Dy | [55] |
| [(Ni(L19)2}2Ln(H2O)Fe(CN)6]2 | N3O2 | O9 | 177.8–179.3 | Y, Gd, Tb | [55] |
| [(Ni(L19)2}2Ln(H2O)Co(CN)6]2 | N3O2 | O9 | 177.8 | Dy | [55] |
| [(Ni(L19)2}2Ln(H2O)W(CN)8]2 | N3O2 | O9 | 175.2/177.1 i | Tb, Dy, Y | [56] |
| [(Ni(L19)2}2Ln(H2O)Co(CN)6]2 | N3O2 | O9 | 177.6 | Tb | [56] |
| [{(NiL31)2Ln(NO3)3}pyz]n | N3O3 | O9 | 149.6, 153.5 | Dy | [57] |
| [{(NiL24)2Ln(H2O)4(oxy-bbz)}(NO3)]n | O6 | N2O8 | 178.8 | Dy | [58] |
| [{[Ni(L32)1.5]2Ln(H2O)5}(CF3SO3)]n | N6 | O7 | 96.7 | Tb | [59] |
| [{[Ni(L32)1.5)]2Ln(H2O)4(NO3)]n | N6 | O8 | 99.2, 99.3 | Gd, Tb | [59] |
| [{[Ni(L33)1.5]2Ln(H2O)5}(NO3)]n | N6 | O7 | 99.8 | Tb | [60] |
| [{[Ni(L33)1.5]2Ln(H2O)6}(CF3SO3)]n | N6 | O8 | 72.8, 73.1 | Tb, Dy | [60] |
| [{(Ni(H2L34))2Ln(H2O)3}(ClO4)3]n | N4 | O9 | 179.2–179.4 | Pr, Sm, Gd | [61] |
| [{(Ni(L35)1.5)2Ln(HL35)(dmf)4}(ClO4)4]n | N4O2 | O8 | 111.5, 111.9 | Gd, Tb | [62] |
| [{(Ni(L35)2)2Ln(dmf)4}(ClO4)3]n | N4O2 | O8 | 115.1 | Dy | [62] |
| [{Ni(L36)1.5}2Ln(dpds)2(H2O)4]n | N2O4 | O9 | 145.7 | Eu | [63] |
| [{Ni(L37)2.5(N3)(H2O)}2Ln(H2O)]n | N3O3 | O9 | 84.9–131.8 | Gd, Pr, Nd | [64,65] |
| Complex | JNiLn (cm−1) | JNiNi (cm−1) | gNi/gLn | D (cm−1) | Ref |
|---|---|---|---|---|---|
| [(NiL1)2Gd](ClO4) | +0.375 | 2.04 | [17] | ||
| [(NiL2)2Gd(NO3)] | +0.19 | 2.24 | +2.1 | [19,20] | |
| [(NiL3)2Gd](NO3) | 0.91 | 1.98 | 4.5 | [21] | |
| [(NiL4)2Gd](NO3) a | 0.64 | 2.04 | [22] | ||
| [(NiL7)2Gd)](ClO4) | 1.02 | 2.01 | [26] | ||
| [(NiL9)2Y(solv)x](ClO4) b | −0.294 | 2.09 | 1.933 | [28] | |
| [(NiL9)2Lu(solv)x](ClO4) c | −0.129 | 2.18 | 2.838 | [28] | |
| [(NiL9)2Gd(solv)x](ClO4) d | −0.009 | −0.377 | 2.102/1.974 | [28] | |
| [(NiL113)2Gd](NO3) | +0.54 | 2.01/2.01 | [30,31] | ||
| [(NiL113)2La](NO3) | +0.46 | 2.245 | +4.91 | [30,31] | |
| [{Ni(HL12)2}2La(NO3)](NO3)2 | −0.978 | 2.177 | 3.133 | [32] | |
| [{Ni(H3L13)2}2Gd](NO3)3 | +0.67 | 2.117 | 4.92 | [33] | |
| [{Ni(H3L14)2}2Sm(O2CMe)2](NO3)3 e | −0.37 | 1.97 | [34] | ||
| [{Ni(H3L14)2}2Gd(O2CMe)2](NO3)3 | +0.42 | 1.98/1.98 | +2.95 | [34] | |
| [(NiL17)2La(O2CMe)2(MeOH)2](NO3) | −0.75 | 2.18 | [37,38] | ||
| [(NiL17)2Ce(O2CMe)2(MeOH)2](NO3) | −1.1 | 2.23 | [37,38] | ||
| [(NiL17)2Pr(O2CMe)2(MeOH)2](NO3) | −1.3 | 2.15 | [37,38] | ||
| [{NiL19(H2O)}2Gd(H2O)](trif)3 f | 4.8 | 2.03 | 0.03 | [21,40] | |
| [{Ni(H2L20)(tren)2}2Gd](NO3)3 | −0.083 | 2.03 | [41] | ||
| [{Ni(H2L20)(tren)2}2Lu](NO3)3 | 2.19 | 3.2 | [41] | ||
| [(NiL24)2La(NO3)3(H2O)4] | −0.63 | 2.22 | [45] | ||
| [(NiL24)2Lu(NO3)3(H2O)4] | −0.65 | 2.17 | [45] | ||
| [(NiL24)2Gd(NO3)3(H2O)4] g | +0.79 | 2.20/2.02 | [45] | ||
| [{Ni(piv)3(bpy)}2Gd(NO3)] h | +0.105 | −0.70 | 2.015/2.0 | [48] | |
| [{Ni(piv)3(Hpiv)(MeCN)}2Gd(NO3)] i | 0.44 | −2.25 | 2.0/2.0 | [48] | |
| [{Ni(piv)3(Hpiv)(MeCN)}2La(NO3)] j | −1.0 | 2.24 | [48] | ||
| [Ni2(L29’)3(L29’’)Gd(NO3)(H2O)](ClO4)2 k | +1.03 | 2.246 | [50,51] | ||
| [Ni2(L29’)4Y(NO3)(H2O)][Ln(NO3)5](ClO4)2 l | 0 | 2.15 | [50,51] |
| Complex | U (K) | τ0 (s) | Ref. |
|---|---|---|---|
| [(NiL1)2Dy](ClO4) a | 10.8 | 2.3 × 10−5 | [17] |
| [(NiL4)2Dy](NO3) b | 14.17 | 1.09 × 10−6 | [22] |
| [(NiL4)2Dy](ClO4) b | 11.13 | 6.72 × 10−6 | [22] |
| [{Ni(L19)2}2Dy(H2O)Fe(CN)6]2 b | 25.0 | 1.6 × 10−7 | [54] |
| [(Ni(L19)2}2Tb(H2O)Cr(CN)6]2 b | 21.9 | 4.71 × 10−8 | [55] |
| [(Ni(L19)2}2Dy(H2O)Cr(CN)6]2 b | 38.9 | 4.89 × 10−9 | [55] |
| [(Ni(L19)2}2Dy(H2O)Cr(CN)6]2·2PPPO c | 37.2 | 6.44 × 10−9 | [55] |
| [(Ni(L19)2}2Tb(H2O)Fe(CN)6]2 b | 29.6 | 4.52 × 10−10 | [55] |
| [(Ni(L19)2}2Dy(H2O)Co(CN)6]2 b | 24.4 | 4.94 × 10−7 | [55] |
| [(Ni(L19)2}2Tb(H2O)W(CN)8]2 b | 23.0 | 2.57 × 10−7 | [56] |
| [(Ni(L19)2}2Dy(H2O)W(CN)8]2 b | 26.4 | 6.0 × 10−8 | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raptopoulou, C.P. Heterometallic Complexes Containing the NiII-LnIII-NiII Moiety—Structures and Magnetic Properties. Crystals 2020, 10, 1117. https://doi.org/10.3390/cryst10121117
Raptopoulou CP. Heterometallic Complexes Containing the NiII-LnIII-NiII Moiety—Structures and Magnetic Properties. Crystals. 2020; 10(12):1117. https://doi.org/10.3390/cryst10121117
Chicago/Turabian StyleRaptopoulou, Catherine P. 2020. "Heterometallic Complexes Containing the NiII-LnIII-NiII Moiety—Structures and Magnetic Properties" Crystals 10, no. 12: 1117. https://doi.org/10.3390/cryst10121117
APA StyleRaptopoulou, C. P. (2020). Heterometallic Complexes Containing the NiII-LnIII-NiII Moiety—Structures and Magnetic Properties. Crystals, 10(12), 1117. https://doi.org/10.3390/cryst10121117