Analysis of Optical Performance Degradation in an Ion-Doped Liquid-Crystal Cell with Electrical Circuit Modeling
Abstract
:1. Introduction
2. Measurement Setup and Cell Fabrication
3. Experimental Results and Discussion
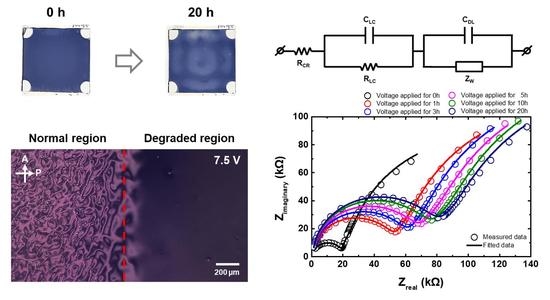
3.1. Change in the Optical Characteristics of the Ion-Doped LC Cell
3.2. Change in the Impedance of the Ion-Doped LC Cell
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Heilmeier, G.H.; Zanoni, L.A. Guest-Host interactions in nematic liquid crystals. A new electrooptic effect. Appl. Phys. Lett. 1968, 13, 91–92. [Google Scholar] [CrossRef]
- Doane, J.W.; Vaz, N.A.; Wu, B.-G.; Zumer, S. Field controlled light scattering from nematic microdroplets. Appl. Phys. Lett. 1986, 48, 269–271. [Google Scholar] [CrossRef]
- Drzaic, P.S. Polymer dispersed nematic liquid crystal for large area displays and light valves. J. Appl. Phys. 1986, 60, 2142–2148. [Google Scholar] [CrossRef]
- Yang, D.-K.; Chien, L.-C.; Doane, J.W. Cholesteric liquid crystal/polymer dispersion for haze-free light shutters. Appl. Phys. Lett. 1992, 60, 3102–3104. [Google Scholar] [CrossRef] [Green Version]
- Granqvist, C.G.; Wittwer, V. Materials for solar energy conversion: An overview. Sol. Energy Mater. Sol. Cells 1998, 54, 39–48. [Google Scholar] [CrossRef]
- Azens, A.; Granqvist, C. Electrochromic smart windows: Energy efficiency and device aspects. J. Solid State Electrochem. 2003, 7, 64–68. [Google Scholar] [CrossRef]
- Vergaz, R.; Sánchez-Pena, J.-M.; Barrios, D.; Vazquez, M.C.; Contreras-Lallana, P. Modelling and electro-optical testing of suspended particle devices. Sol. Energy Mater. Sol. Cells 2008, 92, 1483–1487. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-T.; Lin, T.-H. Bistable reflective polarizer-free optical switch based on dye-doped cholesteric liquid crystal. Opt. Mater. Express 2011, 1, 1457–1462. [Google Scholar] [CrossRef]
- Yu, B.-H.; Huh, J.-W.; Heo, J.; Yoon, T.-H. Simultaneous control of haze and transmittance using a dye-doped cholesteric liquid crystal cell. Liq. Cryst. 2015, 42, 1460–1464. [Google Scholar] [CrossRef]
- Jensen, J.; Hösel, M.; Dyer, A.L.; Krebs, F.C. Development and Manufacture of Polymer-Based Electrochromic Devices. Adv. Funct. Mater. 2015, 25, 2073–2090. [Google Scholar] [CrossRef]
- Barrios, D.; Vergaz, R.; Sánchez-Pena, J.M.; García-Cámara, B.; Granqvist, C.G.; Niklasson, G.A. Simulation of the thickness dependence of the optical properties of suspended particle devices. Sol. Energy Mater. Sol. Cells 2015, 143, 613–622. [Google Scholar] [CrossRef]
- Lorenz, A.; Omairat, F.; Braun, L.; Kolosova, V. Nematic copolymer network LCs for swift continuous phase modulation and opaque scattering states. Mol. Cryst. Liq. Cryst. 2017, 646, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Choi, T.-H.; Woo, J.-H.; Baek, J.-M.; Choi, Y.; Yoon, T.-H. Fast Control of Haze Value Using Electrically Switchable Diffraction in a Fringe-Field Switching Liquid Crystal Device. IEEE Trans. Electron Devices 2017, 64, 3213–3218. [Google Scholar] [CrossRef]
- Zhan, Y.; Schenning, A.P.H.J.; Broer, D.J.; Zhou, G.; Liu, D. Light-Driven Electrohydrodynamic Instabilities in Liquid Crystals. Adv. Funct. Mater. 2018, 28, 1707436. [Google Scholar] [CrossRef] [Green Version]
- Huh, J.-W.; Kim, J.-H.; Oh, S.-W.; Ji, S.-M.; Yoon, T.-H. Ion-doped liquid-crystal cell with low opaque-state specular transmittance based on electro-hydrodynamic effect. Dye. Pigment. 2018, 150, 16–20. [Google Scholar] [CrossRef]
- Choi, T.-H.; Woo, J.-H.; Jeon, B.-G.; Kim, J.; Cha, M.; Yoon, T.-H. Fast fringe-field switching of vertically aligned liquid crystals between high-haze translucent and haze-free transparent states. Liq. Cryst. 2018, 45, 1419–1427. [Google Scholar] [CrossRef]
- Jeon, B.-G.; Choi, T.-H.; Do, S.-M.; Woo, J.-H.; Yoon, T.-H. Effects of Curing Temperature on Switching Between Transparent and Translucent States in a Polymer-Stabilized Liquid-Crystal Cell. IEEE Trans. Electron Devices 2018, 65, 4387–4393. [Google Scholar] [CrossRef]
- Choi, Y.; Oh, S.-W.; Choi, T.-H.; Yu, B.-H.; Yoon, T.-H. Formation of polymer structure by thermally-induced phase separation for a dye-doped liquid crystal light shutter. Dye. Pigment. 2019, 163, 749–753. [Google Scholar] [CrossRef]
- Huh, J.-W.; Seo, J.-H.; Oh, S.-W.; Kim, S.-H.; Yoon, T.-H. Tristate switching of a liquid-crystal cell among initial transparent, haze-free dark, and high-haze dark states. J. Mol. Liq. 2019, 281, 81–85. [Google Scholar] [CrossRef]
- Oh, S.-W.; Jeon, B.-G.; Choi, T.-H.; Do, S.-M.; Yoon, T.-H. Independent control of haze and total transmittance with a dye-doped liquid crystal phase-grating device. Appl. Opt. 2019, 58, 4315–4319. [Google Scholar] [CrossRef]
- Perlmutter, S.H.; Doroski, D.; Moddel, G. Degradation of liquid crystal device performance due to selective adsorption of ions. Appl. Phys. Lett. 1996, 69, 1182–1184. [Google Scholar] [CrossRef]
- Costa, M.R.; Altafim, R.A.C.; Mammana, A.P. Ionic impurities in nematic liquid crystal displays. Liq. Cryst. 2001, 28, 1779–1783. [Google Scholar] [CrossRef]
- Xu, D.; Peng, F.; Chen, H.; Yuan, J.; Wu, S.-T.; Li, M.-C.; Lee, S.-L.; Tsai, W.-C. Image sticking in liquid crystal displays with lateral electric fields. J. Appl. Phys. 2014, 116, 193102. [Google Scholar] [CrossRef] [Green Version]
- Heilmeier, G.H.; Zanoni, L.A.; Barton, L.A. Dynamic scattering: A new electrooptic effect in certain classes of nematic liquid crystals. Proc. IEEE 1968, 56, 1162–1171. [Google Scholar] [CrossRef]
- Heilmeier, G.H.; Zanoni, L.A.; Barton, L.A. Further studies of the dynamic scattering mode in nematic liquid crystals. IEEE Trans. Electron Dev. 1970, 17, 22–26. [Google Scholar] [CrossRef]
- Baise, A.; Teucher, I.; Labes, M.M. Effect of charge-transfer acceptors on dynamic scattering in a nematic liquid crystal. Appl. Phys. Lett. 1972, 21, 142–143. [Google Scholar] [CrossRef]
- Lim, H.S.; Margerum, J.D.; Graube, A. Electrochemical Properties of Dopants and the D-C Dynamic Scattering of a Nematic Liquid Crystal. J. Electrochem. Soc. 1977, 124, 1389–1394. [Google Scholar] [CrossRef]
- Blinov, L.M.; Chigrinov, V.G. Electrooptic Effects in Liquid Crystal Materials, 1st ed.; Springer New York: New York, NY, USA, 1996; pp. 235–307. [Google Scholar]
- Cho, Y.-K.; Granick, S. A surface forces platform for dielectric measurements. J. Chem. Phys. 2003, 119, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Barbero, G.; Alexe-Ionescu, A.L. Role of the diffuse layer of the ionic charge on the impedance spectroscopy of a cell of liquid. Liq. Cryst. 2005, 32, 943–949. [Google Scholar] [CrossRef]
- Alexe-Ionescu, A.L.; Barbero, G.; Lelidis, I. Models for ionic contribution to the complex dielectric constant of nematic liquid crystals. Phys. Rev. E 2009, 80, 061203. [Google Scholar] [CrossRef]
- Belyaev, B.A.; Drokin, N.A.; Maslennikov, A.N. Impedance spectroscopy investigation of liquid crystals doped with ionic surfactants. Phys. Solid State 2014, 56, 1455–1462. [Google Scholar] [CrossRef]
- Belyaev, B.A.; Drokin, N.A. Impedance spectroscopy investigation of electrophysical characteristics of the electrode-liquid crystal interface. Phys. Solid State 2015, 57, 181–187. [Google Scholar] [CrossRef]
- Urbanski, M.; Lagerwall, J.P.F. Nanoparticles dispersed in liquid crystals: Impact on conductivity, low-frequency relaxation and electro-optical performance. J. Mater. Chem. C 2016, 4, 3485–3491. [Google Scholar] [CrossRef]
- Dalir, N.; Javadian, S.; Kakemam, J.; Yousefi, A. Evolution of electro-chemical and electro-optical properties of nematic liquid crystal doped with graphene oxide. J. Mol. Liq. 2018, 265, 398–407. [Google Scholar] [CrossRef]
- Joets, A.; Ribotta, R. Hydrodynamic transitions to chaos in the convection of an anisotropic fluid. J. Phys. 1986, 47, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Kai, S.; Zimmermann, W.; Andoh, M.; Chizumi, N. Local transition to turbulence in electrohydrodynamic convection. Phys. Rev. Lett. 1990, 64, 1111–1114. [Google Scholar] [CrossRef]
- Sprokel, G.J. Resistivity, Permittivity and the Electrode Space Charge of Nematic Liquid Crystals. Mol. Cryst. Liq. Cryst. 1973, 22, 249–260. [Google Scholar] [CrossRef]
- Murakami, S.; Iga, H.; Naito, H. Dielectric properties of nematic liquid crystals in the ultralow frequency regime. J. Appl. Phys. 1996, 80, 6396–6400. [Google Scholar] [CrossRef]
- Costa, M.; Altafim, R.; Mammana, A. Electrical modeling of liquid crystal displays-LCDs. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 204–210. [Google Scholar] [CrossRef]
- Garbovskiy, Y.; Glushchenko, A. Frequency-dependent electro-optics of liquid crystal devices utilizing nematics and weakly conducting polymers. Adv. Opt. Technol. 2018, 7, 243–248. [Google Scholar] [CrossRef]
- Podgornov, F.V.; Gavrilyak, M.; Karaawi, A.; Boronin, V.; Haase, W. Mechanism of electrooptic switching time enhancement in ferroelectric liquid crystal/gold nanoparticles dispersion. Liq. Cryst. 2018, 45, 1594–1602. [Google Scholar] [CrossRef]
- Mada, H.; Ryuzaki, M. Ion Influence on Nematic Liquid Crystal Cell Impedance at Low Frequency. Jpn. J. Appl. Phys. 1995, 34, 1134–1136. [Google Scholar] [CrossRef]
- Serghei, A.; Tress, M.; Sangoro, J.R.; Kremer, F. Electrode polarization and charge transport at solid interfaces. Phys. Rev. B 2009, 80, 184301. [Google Scholar] [CrossRef]









| Time | RCR (Ω) | RLC (kΩ) | CLC (nF) | CDL (nF) | WSR (MΩ∙s−1/2) | WSC (Ω∙s−1/2) |
|---|---|---|---|---|---|---|
| 0 h | 404.7 | 18.48 | 2.447 | 70.41 | 0.7173 | 0.1744 |
| 1 h | 401.9 | 51.25 | 2.452 | 55.51 | 0.8184 | 0.1952 |
| 3 h | 372.5 | 59.47 | 2.467 | 53.45 | 0.8528 | 0.2039 |
| 5 h | 420.8 | 67.11 | 2.473 | 50.21 | 0.8721 | 0.2067 |
| 10 h | 414.8 | 73.66 | 2.477 | 48.49 | 0.8930 | 0.2086 |
| 20 h | 498.9 | 78.24 | 2.460 | 47.77 | 0.8973 | 0.2095 |
| Time | µ (10−9∙m2∙s−1∙V−1) | D (10−12∙m2∙s−1) | δN (µm) | ns (1018∙m−3) |
|---|---|---|---|---|
| 0 h | 15.381 | 401.79 | 3.4958 | 12.828 |
| 1 h | 6.6649 | 174.11 | 2.5756 | 17.081 |
| 3 h | 6.0044 | 156.85 | 2.5533 | 17.272 |
| 5 h | 5.6785 | 148.34 | 2.5166 | 17.366 |
| 10 h | 5.1490 | 134.51 | 2.4193 | 17.812 |
| 20 h | 4.8520 | 126.75 | 2.3582 | 18.260 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, J.-H.; Huh, J.-W.; Sohn, H.-J.; Lim, E.; Yoon, T.-H. Analysis of Optical Performance Degradation in an Ion-Doped Liquid-Crystal Cell with Electrical Circuit Modeling. Crystals 2020, 10, 55. https://doi.org/10.3390/cryst10020055
Seo J-H, Huh J-W, Sohn H-J, Lim E, Yoon T-H. Analysis of Optical Performance Degradation in an Ion-Doped Liquid-Crystal Cell with Electrical Circuit Modeling. Crystals. 2020; 10(2):55. https://doi.org/10.3390/cryst10020055
Chicago/Turabian StyleSeo, Jeong-Ho, Jae-Won Huh, Ho-Jin Sohn, Eunjung Lim, and Tae-Hoon Yoon. 2020. "Analysis of Optical Performance Degradation in an Ion-Doped Liquid-Crystal Cell with Electrical Circuit Modeling" Crystals 10, no. 2: 55. https://doi.org/10.3390/cryst10020055
APA StyleSeo, J. -H., Huh, J. -W., Sohn, H. -J., Lim, E., & Yoon, T. -H. (2020). Analysis of Optical Performance Degradation in an Ion-Doped Liquid-Crystal Cell with Electrical Circuit Modeling. Crystals, 10(2), 55. https://doi.org/10.3390/cryst10020055