N/N Bridge Type and Substituent Effects on Chemical and Crystallographic Properties of Schiff-Base (Salen/Salphen) Niii Complexes
Abstract
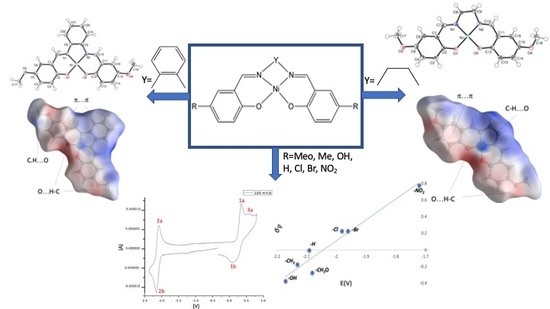
:1. Introduction
2. Materials and Methods
2.1. Abbreviation

2.2. Synthesis of Schiff Base Ligands
2.3. Synthesis of Nickel Complexes
2.4. X-Ray Crystallography
2.5. Cyclic Voltammetry
3. Results and Discussion
3.1. Electronic Spectra
3.2. X-Ray
3.3. Hirshfeld Surface Analysis
3.4. Cyclic Voltammetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ito, Y.N.; Katsuki, T. Asymmetric Catalysis of New Generation Chiral Metallosalen Complexes. Bull. Chem. Soc. Jpn. 1999, 72, 603–619. [Google Scholar] [CrossRef]
- Naeimi, H.; Karshenas, A. Highly regioselective conversion of epoxides to β-hydroxy nitriles using metal(II) Schiff base complexes as new catalysts under mild conditions. Polyhedron 2013, 49, 234–238. [Google Scholar] [CrossRef]
- Dalton, C.T.; Ryan, K.M.; Langan, I.J.; Coyne, É.J.; Gilheany, D.G. Asymmetric alkene epoxidation with chromium oxo salen complexes: Effect of π-rich and other types of additives. J. Mol. Catal. A Chem. 2002, 187, 179–187. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, L.; Su, W.; Yang, Q.; Li, C. Asymmetric ring-opening of epoxides on chiral Co(Salen) catalyst synthesized in SBA-16 through the “ship in a bottle” strategy. J. Catal. 2007, 248, 204–212. [Google Scholar] [CrossRef]
- Bordoloi, A.; Amrute, A.P.; Halligudi, S.B. [Ru(salen)(NO)] complex encapsulated in mesoporous SBA-16 as catalyst for hydrogenation of ketones. Catal. Commun. 2008, 10, 45–48. [Google Scholar] [CrossRef]
- Chatterjee, D.; Bajaj, H.C.; Das, A.; Bhatt, K. First report on highly efficient alkene hydrogenation catalysed by Ni(salen) complex encapsulated in zeolite. J. Mol. Cat. 1994, 92, L235–L238. [Google Scholar] [CrossRef]
- Hamdan, H.; Navijanti, V.; Nur, H.; Muhid, M.N.M. Fe(III)-salen encapsulated Al-MCM-41 as a catalyst in the polymerisation of bisphenol-A. J. Solid State Sci. 2005, 7, 239–244. [Google Scholar] [CrossRef]
- Ding, L.; Liang, S.; Zhang, J.; Ding, C.; Chen, Y.; Lü, X. Cu2+-templated self-assembly of an asymmetric Salen-Cu(II) complex and its application in catalytic polymerization of methyl methacrylate (MMA). Inorg. Chem. Commun. 2014, 44, 173–176. [Google Scholar] [CrossRef]
- Butsch, K.; Günther, T.; Klein, A.; Stirnat, K.; Berkessel, A.; Neudörfl, J. Redox chemistry of copper complexes with various salen type ligands. Inorganica Chimi. Acta 2013, 394, 237–246. [Google Scholar] [CrossRef]
- Pratt, R.C.; Stack, T.D.P. Mechanistic insights from reactions between copper(II)-phenoxyl complexes and substrates with activated C-H bonds. Inorg. Chem. 2005, 44, 2367–2375. [Google Scholar] [CrossRef]
- Thomas, F. Ten years of a biomimetic approach to the copper(II) radical site of galactose oxidase. Eur. J. Inorg. Chem. 2007, 2379–2404. [Google Scholar] [CrossRef]
- Mahapatra, P.; Drew, M.G.B.; Ghosh, A. Ni(II) Complex of N2O3 Donor Unsymmetrical Ligand and Its Use for the Synthesis of NiII-MnII Complexes of Diverse Nuclearity: Structures, Magnetic Properties, and Catalytic Oxidase Activities. Inorg. Chem. 2018, 57, 8338–8353. [Google Scholar] [CrossRef] [PubMed]
- Kianfar, A.H.; Paliz, M.; Roushani, M.; Shamsipur, M. Synthesis, spectroscopy, electrochemistry and thermal study of vanadyl tridentate Schiff base complexes. Spectrochim Acta A 2011, 82, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.C.; Vilas-boas, M.; Piedade, M.F.M.; Freire, C.; Duarte, M.T.; Castro, B. De Electrochemical and X-ray studies of nickel (II) Schiff base complexes derived from salicylaldehyde. Structural effects of bridge substituents on the stabilisation of the +3 oxidation state. Polyhedron 2000, 19, 655–664. [Google Scholar] [CrossRef]
- Shehata, E.E.; Masoud, M.S.; Khalil, E.A.; Abdel-Gaber, A.M. Synthesis, spectral and electrochemical studies of some Schiff base N2O2 complexes. J. Mol. Liq. 2014, 194, 149–158. [Google Scholar] [CrossRef]
- Carradori, S.; De Monte, C.; D’Ascenzio, M.; Secci, D.; Celik, G.; Ceruso, M.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Salen and tetrahydrosalen derivatives act as effective inhibitors of the tumor-associated carbonic anhydrase XII—A new scaffold for designing isoform-selective inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 6759–6763. [Google Scholar] [CrossRef]
- Hang, Z.X.; Dong, B.; Wang, X.W. Synthesis, crystal structures, and antibacterial activity of zinc(II) complexes with bis-schiff bases. Synth. React. Inorg. Met. Org. Chem. 2012, 42, 1345–1350. [Google Scholar] [CrossRef]
- Fasina, T.M.; Ogundele, O.; Ejiah, F.N.; Dueke-Eze, C.U. Biological Activity of Copper (II), Cobalt (II) and Nickel (II) Complexes of Schiff Base Derived from O-phenylenediamine and 5-bromosalicylaldehyde. Int. J. Biol. Chem. 2012, 6, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Woldemariam, G.A.; Mandal, S.S. Iron(III)-salen damages DNA and induces apoptosis in human cell via mitochondrial pathway. J. Inorg. Biochem. 2008, 102, 740–747. [Google Scholar] [CrossRef]
- Ansari, K.I.; Grant, J.D.; Kasiri, S.; Woldemariam, G.; Shrestha, B.; Mandal, S.S. Manganese(III)-salens induce tumor selective apoptosis in human cells. J. Inorg. Biochem. 2009, 103, 818–826. [Google Scholar] [CrossRef]
- Meshkini, A.; Yazdanparast, R. Chemosensitization of human leukemia K562 cells to taxol by a Vanadium-salen complex. Exp. Mol. Pathol. 2010, 89, 334–342. [Google Scholar] [CrossRef]
- Immel, T.A.; Grützke, M.; Batroff, E.; Groth, U.; Huhn, T. Cytotoxic dinuclear titanium-salan complexes: Structural and biological characterization. J. Inorg. Biochem. 2012, 106, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Gravert, D.J.; Griffin, J.H. Specific DNA cleavage mediated by manganese complex [Salen Mn(III)]+. J. Org. Chem. 1993, 58, 820–822. [Google Scholar] [CrossRef]
- Peng, B.; Zhou, W.H.; Yan, L.; Liu, H.W.; Zhu, L. DNA-binding and cleavage studies of chiral Mn(III) salen complexes. Transit. Metal. Chem. 2009, 34, 231–237. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Mandal, S.S. Ambient oxygen activating water soluble Cobalt-Salen complex for DNA cleavage. J. Chem. Soc. Chem. Commun. 1995, 2489–2490. [Google Scholar] [CrossRef]
- Ali, A.; Kamra, M.; Bhan, A.; Mandal, S.S.; Bhattacharya, S. New Fe(III) and Co(II) salen complexes with pendant distamycins: Selective targeting of cancer cells by DNA damage and mitochondrial pathways. Dalton Trans. 2016, 45, 9345–9353. [Google Scholar] [CrossRef]
- Muller, J.G.; Paikoff, S.J.; Rokita, S.E.; Burrows, C.J. DNA modification promoted by water-soluble nickel(II) salen complexes: A switch to DNA alkylation. J. Inorg. Biochem. 1994, 54, 199–206. [Google Scholar] [CrossRef]
- Mariappan, M.; Suenaga, M.; Mukhopadhyay, A.; Maiya, B.G. Synthesis, structure, DNA binding and photonuclease activity of a nickel(II) complex with a N,N′-Bis(salicylidene)-9-(3,4-diaminophenyl) acridine ligand. Inorg. Chim. Acta 2012, 390, 95–104. [Google Scholar] [CrossRef]
- Zhou, C.Q.; Liao, T.C.; Li, Z.Q.; Gonzalez-Garcia, J.; Reynolds, M.; Zou, M.; Vilar, R. Dinickel–salphen complexes as binders of human telomeric dimeric G-quadruplexes. Chem. Eur. J. 2017, 23, 4713–4722. [Google Scholar] [CrossRef]
- Reed, J.E.; Arnal, A.A.; Neidle, S.; Vilar, R. Stabilization of G-quadruplex DNA and inhibition of telomerase activity by square-planar nickel(II) complexes. J. Am. Chem. Soc. 2006, 128, 5992–5993. [Google Scholar] [CrossRef]
- Mukherjee, P.; Biswas, C.; Drew, M.G.B.; Ghosh, A. Structural variations in Ni(II) complexes of salen type di-Schiff base ligands. Polyhedron 2007, 26, 3121–3128. [Google Scholar] [CrossRef]
- Ghaemi, A.; Fayyazi, K.; Keyvani, B.; Ng, S.W.; Tiekink, E.R.T. {2,2′-[o-Phenylenebis(nitrilomethanylylidene)]diphenolato- κ4 O,N,N′,O′}nickel(II) monohydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, E67, m1481–m1482. [Google Scholar] [CrossRef] [Green Version]
- Siegler, M.A.; Lutz, M. Ni(salen): A system that forms many solvates with interacting Ni atoms. Cryst. Growth Des. 2009, 9, 1194–1200. [Google Scholar] [CrossRef]
- Choudhary, A.; Das, B.; Ray, S. Enhanced catalytic activity and magnetization of encapsulated nickel Schiff-base complexes in zeolite-Y: A correlation with the adopted non-planar geometry. Dalton Trans. 2016, 45, 18967–18976. [Google Scholar] [CrossRef]
- Xu, Y.; Xue, L.; Wang, Z.G. Synthesis, X-ray crystal structures, and antibacterial activities of Schiff base nickel(II) complexes with similar tetradentate Schiff bases. Russ. J. Coord. Chem. 2017, 43, 314–319. [Google Scholar] [CrossRef]
- Lamour, E.; Routier, S.; Bernier, J.L.; Catteau, J.P.; Bailly, C.; Vezin, H. Oxidation of Cu(II) to Cu(III), free radical production, and DNA cleavage by hydroxy-salen-copper complexes. Isomeric effects studied by ESR and electrochemistry. J. Am. Chem. Soc. 1999, 121, 1862–1869. [Google Scholar] [CrossRef]
- Aein Jamshid, K.; Asadi, M.; Hossein Kianfar, A. Synthesis, characterization and thermal studies of dinuclear adducts of diorganotin(IV) dichlorides with nickel(II) Schiff-base complexes in chloroform. J. Coord. Chem. 2009, 62, 1187–1198. [Google Scholar] [CrossRef]
- Biradar, N.S.; Karajagi, G.V.; Roddabasanagoudar, V.L.; Aminabhavi, T.M. Geometrical transformations around nickel(ii) with silicon(iv) tetrachloride. Synth. React. Inorg. Met.-Org. Chem. 1984, 14, 773–783. [Google Scholar] [CrossRef]
- Naeimi, H.; Moradian, M. Efficient synthesis and characterization of some novel nitro-schiff bases and their complexes of nickel(II) and copper(II). J. Chem. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Eltayeb, N.E.; Teoh, S.G.; Chantrapromma, S.; Fun, H.K.; Ibrahim, K. 4,4′-Dimethoxy-2,2′-[1,2-phenylene-bis(nitrilomethylidyne)] diphenol. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63. [Google Scholar] [CrossRef]
- Wang, J.; Bei, F.; Xu, X.; Yang, X.; Wang, X. Crystal structure and characterization of 1, 2-N, N-disallicydene-phenylamineato nickel (II) complex. J. Chem. Crystallogr. 2003, 33, 845–849. [Google Scholar] [CrossRef]
- Elerman, Y.; Elmali, A.; Kabak, M.; Aydin, M.; Peder, M. Crystal structure of bis-N,N’-p-chloro-salicylideneamine-1,2.diaaminobenzene. J. Chem. Cryst. 1994, 24, 603–606. [Google Scholar] [CrossRef]
- Batley, G.; Graddon, D. Nickel(II) hydrosalicylamide complexes. Aust. J. Chem. 1967, 20, 1749. [Google Scholar] [CrossRef] [Green Version]
- Kondo, M.; Nabari, K.; Horiba, T.; Irie, Y.; Shimizu, Y.; Fuwa, Y. Synthesis and crystal structure of [Ni{bis(2,5-dihydroxysalicylidene)ethylenediaminato}]: A hydrogen bonded assembly of Ni (II)–salen complex. Inorga Chem Commun 2003, 6, 154–156. [Google Scholar] [CrossRef]
- Asadi, M.; Jamshid, K.A.; Kyanfar, A.H. Synthesis, characterization and equilibrium study of the dinuclear adducts formation between nickel(II) Salen-type complexes with diorganotin(IV) dichlorides in chloroform. Inorganica Chimica Acta 2007, 360, 1725–1730. [Google Scholar] [CrossRef]
- Oxford Diffraction Ltd. Available online: https://www.rigaku.com/products/smc/crysalis (accessed on 17 July 2019).
- Clark, R.C.; Reid, J.S. The analytical calculation of absorption in multifaceted crystals. Acta Crystallogr. A 1995, 51, 887–897. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Garg, B.S.; Nandan Kumar, D. Spectral studies of complexes of nickel(II) with tetradentate schiff bases having N2O2 donor groups. Spectrochim Acta A Mol. Biomol. Spectrosc. 2003, 59, 229–234. [Google Scholar] [CrossRef]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: New York, NY, USA, 1984. [Google Scholar]
- Maki, G. Ligand field theory of Ni (II) complexes. I. Electronic energies and singlet ground-state conditions of Ni (II) complexes of different symmetries. J. Chem. Phys. 1958, 28, 651–662. [Google Scholar] [CrossRef]
- Kianfar, A.H.; Dostani, M.; Mahmood, W.A.K. An unprecedented DDQ-nickel(II)Salen complex interaction and X-ray crystal structure of nickel(II)Salen.DDH co-crystal. Polyhedron 2015, 85, 488–492. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. Cryst. Eng. Comm. 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Available online: http://hirshfeldsurface.net/CrystalExplorer (accessed on 17 July 2019).
- Isse, A.A.; Gennaro, A.; Vianello, E. Electrochemical reduction of Schiff base ligands H2salen and H2salophen. Electrochim. Acta 1997, 42, 2065–2071. [Google Scholar] [CrossRef]
- Pooyan, M.; Ghaffari, A.; Behzad, M.; Amiri Rudbari, H.; Bruno, G. Tetradentate N 2O 2 type Nickel(II) Schiff base complexes derived from meso -1,2-diphenyle-1,2-ethylenediamine: Synthesis, characterization, crystal structures, electrochemistry, and catalytic studies. J. Coord. Chem. 2013, 66, 4255–4267. [Google Scholar] [CrossRef]
- Freire, C. Spectroscopic characterisation of electrogenerated nickel(III) species. Complexes with N2O2 Schiff-base ligands derived from salicylaldehyde. J. Chem. Soc. Dalton Trans. 1998, 1491–1498. [Google Scholar] [CrossRef]
- Jäger, E.G.; Schuhmann, K.; Görls, H. Syntheses, characterization, redox behavior and Lewis acidity of chiral nickel(II) and copper(II) Schiff base complexes. Inorg. Chim. Acta 1997, 255, 295–305. [Google Scholar] [CrossRef]
- Zolezzi, S.; Spodine, E.; Decinti, A. Electrochemical studies of copper(II) complexes with Schiff-base ligands. Polyhedron 2002, 21, 55–59. [Google Scholar] [CrossRef]
- Kianfar, A.H.; Sobhani, V.; Dostani, M.; Shamsipur, M.; Roushani, M. Synthesis, spectroscopy, electrochemistry and thermal study of vanadyl unsymmetrical Schiff base complexes. Inorg. Chim. Acta 2011, 365, 108–112. [Google Scholar] [CrossRef]













| Compound | NiMesalen | NiMeOsalen | NiMeOsalphen | Nisalphen |
|---|---|---|---|---|
| Empirical formula | C18 H18 N2 Ni O2 | C18 H18 N2 Ni O4 | C22 H18 N2 Ni O4 | C43 H31 Cl9 N4 Ni2 O4 |
| Formula weight | 353.05 | 385.05 | 433.09 | 1104.19 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Triclinic |
| Space group | C 2/c | P 21/c | P 21/n | P -1 |
| Unit cell dimensions | ||||
| a (Å) | a = 21.422(2) | a = 16.5157(9) | a = 13.5517(4) | a = 13.7180(10) |
| b (Å) | b = 13.1534(18) | b = 7.2835(3) | b = 7.7890(2) | b = 14.030(2) |
| c (Å) | c = 6.5394(6) | c = 13.7886(7) | c = 17.0198(4) | c = 14.908(2) |
| α (°) | α = 90. | α = 90 | α = 90. | α = 115.285(12) |
| β (°) | β = 96.280(9). | β = 104.809(5) | β = 100.197(3). | β = 116.477(11) |
| γ (°) | γ = 90. | γ = 90 | γ = 90. | γ = 92.967(10) |
| Volume(Å3) | 1831.6(4) | 1603.56(14) | 1768.14(8) | 2215.8(5) |
| Z | 4 | 4 | 4 | 2 |
| Density (calculated) (mg/m3) | 1.280 | 1.595 | 1.627 | 1.655 |
| Absorption coefficient (mm−1) | 1.068 | 1.237 | 1.880 | 1.441 |
| F(000) | 736 | 800 | 896 | 1116 |
| Crystal size(mm3) | 0.470 × 0.090 × 0.070 | 0.550 × 0.160 × 0.130 | 0.470 × 0.230 × 0.200 | 0.520 × 0.170 × 0.160 |
| Theta range for data collection | 3.641 to 29.388°. | 3.445 to 29.394°. | 3.854 to 73.599°. | 3.380 to 29.513°. |
| Index ranges | −27 < = h < = 25, −16 < = k < = 16, −5 < = l < = 9 | −20 < = h < = 17, −9 < = k < = 10, −18 < = l < = 19 | −16 < = h < = 16, −7 < = k < = 9, −20 < = l < = 19 | −18 < = h < = 18, −18 < = k < = 18, −19 < = l < = 16 |
| Reflections collected | 4413 | 11931 | 11435 | 18883 |
| Independent reflections | 2132 [R(int) = 0.0441] | 3921 [R(int) = 0.0215] | 3514 [R(int) = 0.0177] | 10314 [R(int) = 0.0282] |
| Completeness to theta = 25.242° | 99.5 % | 99.8 % | 100.0 % | 99.7 % |
| Absorption correction | Analytical | Analytical | Analytical | Analytical |
| Max. and min. transmission | 0.929 and 0.750 | 0.854 and 0.646 | 0.725 and 0.556 | 0.827 and 0.682 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 2132/0/106 | 3921/0/228 | 3514/0/264 | 10314/0/559 |
| Goodness-of-fit on F2 | 1.019 | 1.047 | 1.058 | 1.027 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0506, wR2 = 0.0944 | R1 = 0.0247, wR2 = 0.0644 | R1 = 0.0313, wR2 = 0.0854 | R1 = 0.0394, wR2 = 0.0796 |
| R indices (all data) | R1 = 0.0802, wR2 = 0.1062 | R1 = 0.0287, wR2 = 0.0671 | R1 = 0.0332, wR2 = 0.0870 | R1 = 0.0561, wR2 = 0.0892 |
| Largest diff. peak and hole | 0.601 and −0.625 e.Å−3 | 0.373 and −0.344 e.Å−3 | 0.238 and −0.567 e.Å−3 | 0.914 and −0.815 e.Å−3 |
| Compound | λmax (ε, L mol−1 cm−1) in DMSO | Compound | λmax (ε, L mol−1 cm−1) in DMSO |
|---|---|---|---|
| MeOsalen | 260, (21145), 345(14109) | NiMeOsalen | 258(39143), 330(7681), 431(7096) |
| Mesalen | 260(17555), 326(8568), 427(176) | NiMesalen | 260(47643), 334(7975), 417(6478) |
| OHsalen | 260(13265), 350(8593) | NiOHsalen | 260(40311), 333(7338), 438(6053) |
| Salen | 260(8747), 327(18295), 410(299) | NiSalen | 260(58958), 324(8061), 407(5906) |
| Clsalen | 258(14461), 327(7794), 420(552) | NiClsalen | 258(44337), 324(8216), 415(6263) |
| Brsalen | 260(16272), 327(7479), 419(653) | NiBrsalen | 258(48523), 326(8863), 414(6419) |
| NO2salen | 258(17745), 370(20301), 422(30053) | NiNO2salen | 263(18147), 340(11715), 405(22660) |
| MeOsalphen | 276(42299), 348(18694) | NiMeOsalphen | 268(50128), 296(2969), 382(26461), 511(2355) |
| Mesalphen | 274(18573), 341(14755), 450(790) | NiMesalphen | 260(55055),292(22231), 380(26821), 487(8851), 679(5.6) |
| OHsalphen | 274(20172), 370(13706) | NiOHsalphen | 260(39286), 298(19113), 386(22873), 518(8406) |
| Salphen | 269(22051), 332(18444), 448(1418) | NiSalphen | 260(37709, 298(14534), 377(22632), 475(7305) |
| Clsalphen | 262(18058), 275(16555), 338(8525), 398(1779) | NiClsalphen | 262(59148), 380(27100), 476(10739), 580(179) |
| Brsalphen | 274(18364), 339(2102), 404(3323), 451(3341) | NiBrsalphen | 264(36210), 314(13762), 378(21675) 478(23408), 674(750) |
| NiMesalen | NiMeOsalen | NiMeOsalphen | |||
| Bond * | Lengths | Bond | Lengths | Bond | Lengths |
| C2—O1 | 1.323(3) | C2—O1 | 1.3125(17) | C2—O1 | 1.3077(18) |
| C5—C17 | 1.525(4) | C5—O3 | 1.3770(17) | C5—O3 | 1.3765(18) |
| C7—N1 | 1.297(3) | C7—N1 | 1.2892(19) | C7—N1 | 1.298(2) |
| C8—N1 | 1.469(4) | C8—N1 | 1.4774(17) | C8—C9 | 1.397(2) |
| C8—C8#1 | 1.515(5) | C8—C9 | 1.504(2) | C8—N1 | 1.4233(19) |
| O1—Ni1 | 1.852(2) | C17—O3 | 1.4256(18) | C17—O3 | 1.4219(19) |
| Ni1—N1#1 | 1.844(2) | O1—Ni1 | 1.8505(10) | N1—Ni1 | 1.8626(12) |
| Ni1—N1 | 1.844(2) | O2—Ni1 | 1.8544(10) | N2—Ni1 | 1.8643(13) |
| Ni1—N2 | 1.8476(12) | Ni1—O2 | 1.8394(11) | ||
| Ni1—N1 | 1.8520(12) | Ni1—O1 | 1.8536(11) | ||
| Bond | Angles | Bond | Angles | Bond | Angles |
| N1#1—Ni1—N1 | 86.32(15) | N1—C8—C9 | 107.28(11) | C9—C8—N1 | 113.44(13) |
| N1#1—Ni1—O1#1 | 94.95(9) | N2—C9—C8 | 107.04(11) | C8—C9—N2 | 114.08(13) |
| N1—Ni1—O1#1 | 178.40(10) | N2—Ni1—O1 | 179.52(5) | C8—N1—Ni1 | 113.37(10) |
| N1#1—Ni1—O1 | 178.40(10) | N2—Ni1—N1 | 86.03(5) | C9—N2—Ni1 | 113.04(10) |
| N1—Ni1—O1 | 94.95(9) | O1—Ni1—N1 | 94.40(5) | O2—Ni1—O1 | 84.12(5) |
| O1#1—Ni1—O1 | 83.79(13) | N2—Ni1—O2 | 93.77(5) | O2—Ni1—N1 | 179.34(5) |
| O1—Ni1—O2 | 85.80(4) | O1—Ni1—N1 | 95.22(5) | ||
| N1—Ni1—O2 | 179.60(5) | O2—Ni1—N2 | 94.60(5) | ||
| C8—N1—Ni1 | 114.60(9) | O1—Ni1—N2 | 177.72(5) | ||
| C9—N2—Ni1 | 113.22(9) | N1—Ni1—N2 | 86.05(6) | ||
| Nisalphen | |||||
| Molecule A | Molecule B | ||||
| Bond * | Lengths | Bond | Lengths | ||
| C2A-O1A | 1.306(3) | C2B-O1B | 1.306(3) | ||
| C7A-N1A | 1.308(3) | C7B-N1B | 1.299(3) | ||
| C8A-C9A | 1.395(3) | C8B-C9B | 1.389(4) | ||
| C8A-N1A | 1.417(3) | C8B-N1B | 1.427(3) | ||
| C12A-O2A | 1.312(3) | C12B-O2B | 1.309(3) | ||
| O1A-Ni1A | 1.8398(16) | O1B-Ni1B | 1.8370(17) | ||
| O2A-Ni1A | 1.8346(18) | O2B-Ni1B | 1.8360(16) | ||
| N1A-Ni1A | 1.857(2) | N1B-Ni1B | 1.8567(19) | ||
| N2A-Ni1A | 1.8564(19) | N2B-Ni1B | 1.856(2) | ||
| Bond | Angles | Bond | Angles | ||
| C9A-C8A-N1A | 113.8(2) | C9B-C8B-N1B | 113.8(2) | ||
| C8A-C9A-N2A | 113.8(2) | C8B-C9B-N2B | 113.8(2) | ||
| C8A-N1A-Ni1A | 113.13(16) | C8B-N1B-Ni1B | 112.87(16) | ||
| C9A-N2A-Ni1A | 112.97(16) | C9B-N2B-Ni1B | 113.22(16) | ||
| O2A-Ni1A-O1A | 83.05(8) | O2B-Ni1B-O1B | 83.03(7) | ||
| O2A-Ni1A-N2A | 95.17(8) | O2B-Ni1B-N2B | 95.47(8) | ||
| O1A-Ni1A-N2A | 178.13(9) | O1B-Ni1B-N2B | 178.47(8) | ||
| O2A-Ni1A-N1A | 178.42(8) | O2B-Ni1B-N1B | 178.24(9) | ||
| O1A-Ni1A-N1A | 95.46(8) | O1B-Ni1B-N1B | 95.23(8) | ||
| N2A-Ni1A-N1A | 86.32(9) | N2B-Ni1B-N1B | 86.27(9) | ||
| Process | ||||||
|---|---|---|---|---|---|---|
| Compound | ∆E (mV) | ipc/ipa | E1/2 (V) | ∆E (mV) | ipc/ipa | E1/2 (V) |
| NiMeOsalen | 110 | 0.19 | 0.19 | 110 | 0.50 | −2.08 |
| NiMesalen | 240 | 0.31 | 0.17 | 77 | 0.61 | −2.13 |
| NiOHsalen | 200 | 0.12 | 0.073 | 92 | 0.30 | −2.17 |
| Nisalen | 410 | 0.16 | 0.16 | 76 | 0.72 | −2.09 |
| NiClsalen | 390 | 0.16 | 0.22 | 73 | 0.83 | −1.98 |
| NiBrsalen | 400 | 0.23 | 0.21 | 60 | 0.45 | −1.96 |
| NiNO2salen | 200 | 0.41 | 0.33 | 120 | 0.57 | −1.72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novoa-Ramírez, C.S.; Silva-Becerril, A.; Olivera-Venturo, F.L.; García-Ramos, J.C.; Flores-Alamo, M.; Ruiz-Azuara, L. N/N Bridge Type and Substituent Effects on Chemical and Crystallographic Properties of Schiff-Base (Salen/Salphen) Niii Complexes. Crystals 2020, 10, 616. https://doi.org/10.3390/cryst10070616
Novoa-Ramírez CS, Silva-Becerril A, Olivera-Venturo FL, García-Ramos JC, Flores-Alamo M, Ruiz-Azuara L. N/N Bridge Type and Substituent Effects on Chemical and Crystallographic Properties of Schiff-Base (Salen/Salphen) Niii Complexes. Crystals. 2020; 10(7):616. https://doi.org/10.3390/cryst10070616
Chicago/Turabian StyleNovoa-Ramírez, Cynthia S., Areli Silva-Becerril, Fiorella L. Olivera-Venturo, Juan Carlos García-Ramos, Marcos Flores-Alamo, and Lena Ruiz-Azuara. 2020. "N/N Bridge Type and Substituent Effects on Chemical and Crystallographic Properties of Schiff-Base (Salen/Salphen) Niii Complexes" Crystals 10, no. 7: 616. https://doi.org/10.3390/cryst10070616
APA StyleNovoa-Ramírez, C. S., Silva-Becerril, A., Olivera-Venturo, F. L., García-Ramos, J. C., Flores-Alamo, M., & Ruiz-Azuara, L. (2020). N/N Bridge Type and Substituent Effects on Chemical and Crystallographic Properties of Schiff-Base (Salen/Salphen) Niii Complexes. Crystals, 10(7), 616. https://doi.org/10.3390/cryst10070616