Design, Synthesis, and Biological Activity Evaluation of New Donepezil-Like Compounds Bearing Thiazole Ring for the Treatment of Alzheimer’s Disease
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Cholinesterase Enzymes Inhibition Assay
2.3. Kinetic Studies of Enzyme Inhibition
2.4. Inhibition of Beta Amyloid 1–42 (Aβ42) Aggregation
2.5. Prediction of ADME Parameters and BBB Permeability
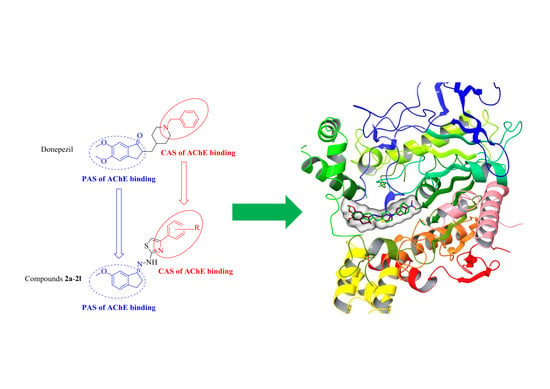
2.6. Molecular Docking
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Synthesis of the Compounds
Synthesis of 2-(6-methoxy-2,3-dihydro-1H-inden-1-ylidene)hydrazine-1-carbothioamide (1)
General Procedure for the Synthesis of Target Compounds (2a–2l)
3.2. Cholinesterase Enzymes Inhibition Assay
3.3. Kinetic Studies of Enzyme Inhibition
3.4. Inhibition of Beta Amyloid 1–42 (Aβ42) Aggregation
3.5. Prediction of ADME Parameters and BBB Permeability
3.6. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sugimoto, H. The new approach in development of anti-Alzheimer’s disease drugs via the cholinergic hypothesis. Chem. Biol. Interact. 2008, 175, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, P.; Cariati, L.; Desiderio, D.; Sgammato, R.; Lamberti, A.; Arcone, R.; Salerno, R.; Nardi, M.; Masullo, M.; Oliverio, M. Design, Synthesis, and Evaluation of Donepezil-Like Compounds as AChE and BACE-1 Inhibitors. ACS Med. Chem. Lett. 2016, 7, 470–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavdar, H.; Senturk, M.; Guney, M.; Durdagi, S.; Kayik, G.; Supuran, C.T.; Ekinci, D. Inhibition of acetylcholinesterase and butyrylcholinesterase with uracil derivatives: Kinetic and computational studies. J. Enzym. Inhib. Med. Chem. 2019, 34, 429–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McHardy, S.F.; Wang, H.-Y.L.; McCowen, S.V.; Valdez, M.C. Recent advances in acetylcholinesterase Inhibitors and Reactivators: An update on the patent literature (2012–2015). Expert Opin. Ther. Pat. 2017, 27, 455–476. [Google Scholar] [CrossRef]
- Rogers, S.L.; Friedhoff, L.T. Long-term efficacy and safety of donepezil in the treatment of Alzheimer’s disease: An interim analysis of the results of a US multicentre open label extension study. Eur. Neuropsychopharmacol. 1998, 8, 67–75. [Google Scholar] [CrossRef]
- Wang, Z.M.; Cai, P.; Liu, Q.H.; Xu, D.Q.; Yang, X.L.; Wu, J.J.; Kong, L.Y.; Wang, X.B. Rational modification of donepezil as multifunctional acetylcholinesterase inhibitors for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2016, 123, 282–297. [Google Scholar] [CrossRef]
- Sağlık, B.N.; Ilgın, S.; Özkay, Y. Synthesis of new donepezil analogues and investigation of their effects on cholinesterase enzymes. Eur. J. Med. Chem. 2016, 124, 1026–1040. [Google Scholar] [CrossRef]
- van Greunen, D.G.; Cordier, W.; Nell, M.; van der Westhuyzen, C.; Steenkamp, V.; Panayides, J.L.; Riley, D.L. Targeting Alzheimer’s disease by investigating previously unexplored chemical space surrounding the cholinesterase inhibitor donepezil. Eur. J. Med. Chem. 2017, 127, 671–690. [Google Scholar] [CrossRef]
- Youssef, K.M.; Fawzy, I.M.; El-Subbagh, H. N-substituted-piperidines as Novel Anti-alzheimer Agents: Synthesis, antioxidant activity, and molecular docking study. Futur. J. Pharm. Sci. 2018, 4, 1–7. [Google Scholar] [CrossRef]
- Silva, D.; Chioua, M.; Samadi, A.; Agostinho, P.; Garção, P.; Lajarín-Cuesta, R.; Ríos, C.D.L.; Iriepa, I.; Moraleda, I.; González-Lafuente, L.; et al. Synthesis, Pharmacological Assessment, and Molecular Modeling of Acetylcholinesterase/Butyrylcholinesterase Inhibitors: Effect against Amyloid-β-Induced Neurotoxicity. ACS Chem. Neurosci. 2013, 4, 547–565. [Google Scholar] [CrossRef] [Green Version]
- Lan, J.-S.; Zhang, T.; Liu, Y.; Yang, J.; Xie, S.-S.; Liu, J.; Miao, Z.-Y.; Ding, Y. Design, synthesis and biological activity of novel donepezil derivatives bearing N -benzyl pyridinium moiety as potent and dual binding site acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2017, 133, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of Human Acetylcholinesterase in Complex with Pharmacologically Important Ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.S.; Tu, W.T.; Luo, M.; Shi, J.G. Molecular Docking and Design of Novel Heterodimers of Donepezil and Huperzine Fragments as Acetylcholinesterase Inhibitors. Chin. J. Struct. Chem. 2016, 35, 839–848. [Google Scholar]
- Vitorović-Todorović, M.D.; Worek, F.; Perdih, A.; Bauk, S.D.; Vujatovic, T.M.; Cvijetic, I.N. The in vitro protective effects of the three novel nanomolar reversible inhibitors of human cholinesterases against irreversible inhibition by organophosphorous chemical warfare agents. Chem. Biol. Interact. 2019, 309, 108714. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Özkay, Ü.D.; Can, Ö.D.; Sağlık, B.N.; Çevik, U.A.; Levent, S.; Özkay, Y.; Ilgin, S.; Atlı, Ö. Design, synthesis, and AChE inhibitory activity of new benzothiazole–piperazines. Bioorg. Med. Chem. Lett. 2016, 26, 5387–5394. [Google Scholar] [CrossRef]
- Acar, Ç.U.; Levent, S.; Sağlık, B.N.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis of Novel 4-(Dimethylaminoalkyl) piperazine-1-carbodithioa te Derivatives as Cholinesterase Inhibitors. Lett. Drug Des. Discov. 2017, 14, 528–539. [Google Scholar]
- Levent, S.; Çevik, U.A.; Sağlık, B.N.; Özkay, Y.; Can, Ö.D.; Özkay, Ü.D.; Uçucu, Ü. Anticholinesterase activity screening of some novel dithiocarbamate derivatives including piperidine and piperazine moieties. Phosphorus Sulfur Silicon Relat. Elem. 2016, 192, 469–474. [Google Scholar] [CrossRef]
- Hussein, W.; Sağlık, B.N.; Levent, S.; Korkut, B.; Ilgın, S.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis and Biological Evaluation of New Cholinesterase Inhibitors for Alzheimer’s Disease. Molecules 2018, 23, 2033. [Google Scholar] [CrossRef] [Green Version]
- Tok, F.; Koçyiğit-Kaymakçıoğlu, B.; Sağlık, B.N.; Levent, S.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis and biological evaluation of new pyrazolone Schiff bases as monoamine oxidase and cholinesterase inhibitors. Bioorg. Chem. 2019, 84, 41–50. [Google Scholar] [CrossRef]
- Cevik, U.A.; Sağlık, B.N.; Levent, S.; Osmaniye, D.; Cavuşoglu, B.K.; Özkay, Y.; Kaplancikli, Z.A. Synthesis and AChE-Inhibitory Activity of New Benzimidazole Derivatives. Molecules 2019, 24, 861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osmaniye, D.; Sağlık, B.N.; Çevik, U.A.; Levent, S.; Çavuşoğlu, B.K.; Özkay, Y.; Kaplancıklı, Z.A.; Turan, G. Synthesis and AChE Inhibitory Activity of Novel Thiazolylhydrazone Derivatives. Molecules 2019, 24, 2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- QikProp, Version 4.8; Schrödinger, LLC: New York, NY, USA, 2016.
- Lipinski, C.A.; Franco, L.; Dominy Beryl, W.; Feeney Paul, J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Duffy, E.M. Prediction of drug solubility from structure. Adv. Drug Deliv. Rev. 2002, 54, 355–366. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunitz, J.D.; Taylor, R. Organic Fluorine Hardly Ever Accepts Hydrogen Bonds. Chem. A Eur. J. 1997, 3, 89–98. [Google Scholar] [CrossRef]
- Dvir, H.; Silman, I.; Harel, M.; Rosenberry, T.L.; Sussman, J.L. Acetylcholinesterase: From 3D structure to function. Chem. Interact. 2010, 187, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Colovic, M.; Krstić, D.; Lazarevic-Pasti, T.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [Green Version]
- Atanasova, M.; Stavrakov, G.; Philipova, I.; Zheleva-Dimitrova, D.; Yordanov, N.; Doytchinova, I. Galantamine derivatives with indole moiety: Docking, design, synthesis and acetylcholinesterase inhibitory activity. Bioorg. Med. Chem. 2015, 23, 5382–5389. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Esteban, G.; Brogi, S.; Shionoya, M.; Wang, L.; Campiani, G.; Unzeta, M.; Inokuchi, T.; Butini, S.; Marco-Contelles, J. Donepezil-like multifunctional agents: Design, synthesis, molecular modeling and biological evaluation. Eur. J. Med. Chem. 2016, 121, 864–879. [Google Scholar] [CrossRef]
- Genest, D.; Rochais, C.; Lecoutey, C.; Jana Sopkova-de Oliveira, S.; Ballandonne, C.; Butt-Gueulle, S.; Legay, R.; Since, M.; Dallemagne, P. Design, synthesis and biological evaluation of novel indano-and thiaindano-pyrazoles with potential interest for Alzheimer’s disease. Med. Chem. Commun. 2013, 4, 925–931. [Google Scholar] [CrossRef]
- Al-Rashid, Z.F.; Hsung, R.P. A computational view on the significance of E-ring in binding of (+)-arisugacin A to acetylcholinesterase. Bioorg. Med. Chem. Lett. 2015, 25, 4848–4853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alipour, M.; Khoobi, M.; Foroumadi, A.; Nadri, H.; Moradi, A.; Sakhteman, A.; Ghandi, M.; Shafiee, A. Novel coumarin derivatives bearing N-benzyl pyridinium moiety: Potent and dual binding site acetylcholinesterase inhibitors. Bioorg. Med. Chem. 2012, 20, 7214–7222. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Gary, E.N.; Shiomi, K.; Rosenberry, T.L. Structures of Human Acetylcholinesterase Bound to Dihydrotanshinone I and Territrem B Show Peripheral Site Flexibility. ACS Med. Chem. Lett. 2013, 4, 1091–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-H.; Yu, L.; Wang, H.; Fang, W.; Ling, X.; Shi, Y.; Lin, C.-T.; Huang, J.-K.; Chang, M.-T.; Chang, C.-S.; et al. Synthesis and Transfer of Single-Layer Transition Metal Disulfides on Diverse Surfaces. Nano Lett. 2013, 13, 1852–1857. [Google Scholar] [CrossRef]
- Vitorović-Todorović, M.D.; Koukoulitsa, C.; Juranic, I.O.; Mandic, L.; Drakulić, B.J. Structural modifications of 4-aryl-4-oxo-2-aminylbutanamides and their acetyl- and butyrylcholinesterase inhibitory activity. Investigation of AChE–ligand interactions by docking calculations and molecular dynamics simulations. Eur. J. Med. Chem. 2014, 81, 158–175. [Google Scholar] [CrossRef] [Green Version]
- Glide, Version 7.1; Schrödinger, LLC: New York, NY, USA, 2016.
- Finkielsztein, L.M.; Castro, E.F.; Fabián, L.E.; Moltrasio, G.Y.; Campos, R.H.; Cavallaro, L.V.; Moglioni, A.G. New 1-indanone thiosemicarbazone derivatives active against BVDV. Eur. J. Med. Chem. 2008, 43, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Maestro, Version 10.6; Schrödinger, LLC: New York, NY, USA, 2016.
- Available online: https://www.schrodinger.com/ (accessed on 20 May 2020).
- LigPrep, Version 3.8; Schrödinger, LLC: New York, NY, USA, 2016.
Sample Availability: Samples of the compounds 2a-2l are available from the authors. |








| Compounds | R1 | R2 |
|---|---|---|
| 2a | –H | –H |
| 2b | –H | –CH3 |
| 2c | –H | –OCH3 |
| 2d | –H | –CN |
| 2e | –H | –NO2 |
| 2f | –H | –F |
| 2g | –H | –CI |
| 2h | –H | –Br |
| 2i | –CH3 | –CH3 |
| 2j | –OCH3 | –OCH3 |
| 2k | –F | –F |
| 2l | –CI | –CI |
| Compounds | AChE % Inhibition | AChE IC50 (µM) | BChE % Inhibition | ||
|---|---|---|---|---|---|
| 1000 µM | 100 µM | 1000 µM | 100 µM | ||
| 2a | 90.28 ± 1.95 | 84.36 ± 1.05 | 0.118 ± 0.004 | 48.12 ± 0.85 | 31.45 ± 0.62 |
| 2b | 85.65 ± 1.20 | 47.38 ± 0.95 | - | 35.20 ± 0.91 | 24.86 ± 0.59 |
| 2c | 88.34 ± 1.00 | 46.26 ± 0.97 | - | 34.45 ± 0.97 | 28.13 ± 0.61 |
| 2d | 80.48 ± 1.34 | 48.71 ± 0.85 | - | 30.50 ± 0.85 | 23.47 ± 0.52 |
| 2e | 98.56 ± 1.85 | 94.14 ± 1.69 | 0.026 ± 0.001 | 46.25 ± 0.90 | 32.75 ± 0.73 |
| 2f | 75.16 ± 1.26 | 43.30 ± 0.90 | - | 35.61 ± 0.72 | 28.45 ± 0.63 |
| 2g | 73.66 ± 0.95 | 41.97 ± 0.97 | - | 29.15 ± 0.76 | 21.74 ± 0.57 |
| 2h | 78.96 ± 1.46 | 46.38 ± 0.89 | - | 32.59 ± 0.84 | 27.21 ± 0.61 |
| 2i | 92.45 ± 1.60 | 86.49 ± 1.24 | 0.070 ± 0.003 | 45.95 ± 0.92 | 30.65 ± 0.80 |
| 2j | 82.50 ± 1.51 | 48.20 ± 0.89 | - | 30.61 ± 0.71 | 24.87 ± 0.69 |
| 2k | 77.36 ± 1.64 | 47.21 ± 0.97 | - | 28.30 ± 0.63 | 21.47 ± 0.71 |
| 2l | 91.28 ± 1.85 | 86.46 ± 1.30 | 0.061 ± 0.002 | 49.32 ± 0.97 | 33.54 ± 0.87 |
| Donepezil | 99.25 ± 2.10 | 97.42 ± 1.89 | 0.021 ± 0.001 | - | - |
| Tacrine | - | - | - | 98.25 ± 1.89 | 95.46 ± 1.34 |
| Comp. | MW | RB | DM | MV | DHB | AHB | PSA | logP | logS | PCaco | logBB | PMDCK | CNS | PM | %HOA | VRF | VRT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2a | 335.42 | 4 | 3.332 | 1083.4 | 1 | 4.25 | 42.958 | 4.743 | −5.854 | 5528.688 | 0.180 | 6206.560 | 1 | 4 | 100 | 0 | 1 |
| 2b | 349.45 | 4 | 2.840 | 1142.4 | 1 | 4.25 | 42.958 | 5.048 | −6.419 | 5528.688 | 0.173 | 6206.560 | 1 | 5 | 100 | 1 | 1 |
| 2c | 360.43 | 5 | 9.732 | 1150.1 | 1 | 5.75 | 68.754 | 3.942 | −6.827 | 1143.337 | −0.664 | 1129.910 | 0 | 4 | 100 | 0 | 1 |
| 2d | 365.45 | 5 | 3.596 | 1151.2 | 1 | 5 | 51.440 | 4.788 | −5.983 | 5528.686 | 0.115 | 6206.559 | 1 | 5 | 100 | 0 | 1 |
| 2e | 380.42 | 5 | 13.462 | 1165.8 | 1 | 5.25 | 91.625 | 4.049 | −6.113 | 568.231 | −1.004 | 530.683 | 2 | 5 | 100 | 0 | 1 |
| 2f | 353.41 | 4 | 5.778 | 1099.6 | 1 | 4.25 | 42.963 | 4.979 | −6.220 | 5528.423 | 0.293 | 10,000 | 1 | 4 | 100 | 0 | 1 |
| 2g | 369.87 | 4 | 5.724 | 1127.6 | 1 | 4.25 | 42.961 | 5.238 | −6.597 | 5528.522 | 0.350 | 10,000 | 1 | 4 | 100 | 1 | 1 |
| 2h | 414.32 | 4 | 5.405 | 1136.5 | 1 | 4.25 | 42.961 | 5.315 | −6.713 | 5528.586 | 0.363 | 10,000 | 1 | 4 | 100 | 1 | 1 |
| 2i | 363.48 | 4 | 3.336 | 1167.2 | 1 | 4.25 | 38.069 | 5.240 | −6.216 | 6852.418 | 0.288 | 7827.314 | 1 | 6 | 100 | 1 | 1 |
| 2j | 395.48 | 6 | 2.543 | 1202.8 | 1 | 5.75 | 55.272 | 4.786 | −5.508 | 5876.146 | 0.100 | 6629.222 | 1 | 6 | 100 | 0 | 0 |
| 2k | 371.40 | 4 | 5.143 | 1108.3 | 1 | 4.25 | 41.521 | 5.129 | −6.404 | 5803.010 | 0.383 | 10,000 | 1 | 4 | 100 | 1 | 1 |
| 2l | 404.31 | 4 | 5.201 | 1140.4 | 1 | 4.25 | 39.548 | 5.444 | −6.460 | 6255.627 | 0.496 | 10,000 | 2 | 4 | 100 | 1 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sağlık, B.N.; Levent, S.; Osmaniye, D.; Acar Çevik, U.; Kaya Çavuşoğlu, B.; Özkay, Y.; Koparal, A.S.; Kaplancıklı, Z.A. Design, Synthesis, and Biological Activity Evaluation of New Donepezil-Like Compounds Bearing Thiazole Ring for the Treatment of Alzheimer’s Disease. Crystals 2020, 10, 637. https://doi.org/10.3390/cryst10080637
Sağlık BN, Levent S, Osmaniye D, Acar Çevik U, Kaya Çavuşoğlu B, Özkay Y, Koparal AS, Kaplancıklı ZA. Design, Synthesis, and Biological Activity Evaluation of New Donepezil-Like Compounds Bearing Thiazole Ring for the Treatment of Alzheimer’s Disease. Crystals. 2020; 10(8):637. https://doi.org/10.3390/cryst10080637
Chicago/Turabian StyleSağlık, Begüm Nurpelin, Serkan Levent, Derya Osmaniye, Ulviye Acar Çevik, Betül Kaya Çavuşoğlu, Yusuf Özkay, Ali Savaş Koparal, and Zafer Asım Kaplancıklı. 2020. "Design, Synthesis, and Biological Activity Evaluation of New Donepezil-Like Compounds Bearing Thiazole Ring for the Treatment of Alzheimer’s Disease" Crystals 10, no. 8: 637. https://doi.org/10.3390/cryst10080637
APA StyleSağlık, B. N., Levent, S., Osmaniye, D., Acar Çevik, U., Kaya Çavuşoğlu, B., Özkay, Y., Koparal, A. S., & Kaplancıklı, Z. A. (2020). Design, Synthesis, and Biological Activity Evaluation of New Donepezil-Like Compounds Bearing Thiazole Ring for the Treatment of Alzheimer’s Disease. Crystals, 10(8), 637. https://doi.org/10.3390/cryst10080637