Fluorinated Tolane Dyads with Alkylene Linkage: Synthesis and Evaluation of Photophysical Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Materials
2.3. Preparation of 1-Trifluoromethyl-2,3,5,6-tetrafluoro-4-[2-(4-methoxymethoxyphenyl)ethyn-1-yl]benzene (4) via an Addition–Elimination Process
1-Trifluoromethyl-2,3,5,6-tetrafluoro-4-[2-(4-methoxymethoxyphenyl)ethyn-1-yl]benzene (4)
2.4. Preparation of 4-(2-(2,3,5,6-Tetrafluoro-4-trifluoromethyl)phenylethyn-1-yl)phenol (5)
4-(2-(2,3,5,6-Tetrafluoro-4-trifluoromethyl)phenylethyn-1-yl)phenol (5)
2.5. Typical Procedure for Preparation of 1,5-Bis[4-{2-(2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenyl)ethyn-1-yl}phenoxy]pentane (2a)
2.5.1. 1,5-Bis[4-{2-(2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenyl)ethyn-1-yl}phenoxy]pentane (2a)
2.5.2. 1,6-Bis[4-{2-(2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenyl)ethyn-1-yl}phenoxy]hexane (2b)
2.5.3. 1,7-Bis[4-{2-(2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenyl)ethyn-1-yl}phenoxy]heptane (2c)
2.5.4. 1,8-Bis[4-{2-(2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenyl)ethyn-1-yl}phenoxy]octane (2d)
2.5.5. 1,9-Bis[4-{2-(2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenyl)ethyn-1-yl}phenoxy]nonane (2e)
2.5.6. 1,10-Bis[4-{2-(2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenyl)ethyn-1-yl}phenoxy]decane (2f)
2.6. X-Ray Crystallography
2.7. Computations
2.8. Photophysical Measurements
3. Results and Discussion
3.1. Theoretical Molecular Assessment
3.2. Synthesis and Crystal Structure
3.3. Photophysical Behavior in a Solution State
3.4. Photophysical Behavior in Crystalline Powder State
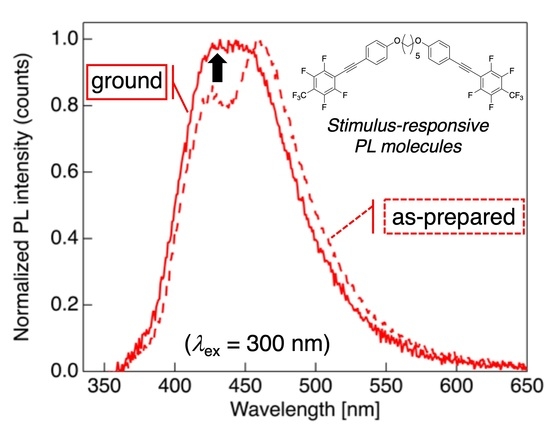
3.5. Mechanochromic PL Behavior of 2a, 2b, and 2d after Mechanical Stimulus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kundu, S.; Sk, B.; Pallavi, P.; Giri, A.; Patra, A. Molecular engineering approaches toward all-organic white light emitting materials. Chem. Eur. J. 2020, 26, 5557–5582. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ho, C.-L.; Wang, L.; Wong, W.-Y. Single-molecular white-light emitters and their potential WOLED applications. Adv. Matter. 2020, 32, 1903269. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Bhagwan, S.; Saini, R.K.; Nishal, V.; Singh, I. Development in organic light-emitting materials and their potential applications. In Advanced Magnetic and Optical Materials; Tiwari, A., Iyer, P.K., Kumar, V., Swart, H., Eds.; Scrivener Publishing: Beverly, MA, USA, 2017; pp. 473–520. [Google Scholar]
- Shen, Q.; Wang, S.; Yang, N.-D.; Zhang, C.; Wu, Q.; Yu, C. Recent development of small-molecule organic fluorophores for multifunctional bioimaging in the second near-infrared window. J. Lumines. 2020, 225, 11738. [Google Scholar] [CrossRef]
- Shaikh, S.; Wang, Y.; ur Rehman, F.; Jiang, H.; Wang, X. Phosphorescent Ir(III) complexes as cellular staining agents for biomedical molecular imaging. Coord. Chem. Rev. 2020, 416, 213344. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, J.; Fang, W.; Yang, L.; Hu, Q.; Wang, Z.; Sun, J.Z.; Tang, B.Z. Sugar-based aggregation-induced emission luminogens: Design, structures, and applications. Chem. Rev. 2020, 120, 4534–4577. [Google Scholar] [CrossRef]
- Yersin, H. (Ed.) Highly Efficient OLEDs: Materials Based on Thermally Activated Delayed Fluorescence; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- Bui, T.-T.; Goubard, F.; Ibrahim-Ouali, M.; Gigmes, D.; Dumur, F. Recent advances on organic blue thermally activated delayed fluorescence (TADF) emitters for organic light-emitting diodes (OLEDs). Beilstein J. Org. Chem. 2018, 14, 282–308. [Google Scholar] [CrossRef] [Green Version]
- Ostroverkhova, O. Organic optoelectronic materials: Mechanisms and applications. Chem. Rev. 2016, 116, 13279–13412. [Google Scholar] [CrossRef]
- Gaspar, D.J.; Polikarpov, E. OLED Fundamentals: Materials, Devices, and Processing of Organic Light-Emitting Diodes; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef]
- Li, H.; Li, B.S.; Tang, B.Z. Molecular design, circularly polarized luminescence, and helical self-assembly of chiral aggregation-induced emission molecules. Chem. Asian J. 2019, 14, 674–688. [Google Scholar] [CrossRef]
- He, Z.; Ke, C.; Tang, B.Z. Journey of aggregation-induced emission research. ACS Omega 2018, 3, 3267–3277. [Google Scholar] [CrossRef] [Green Version]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 21, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; He, B.; Tang, B.Z. Aggregation-induces emission of siloles. Chem. Sci. 2015, 6, 5347–5365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Peng, Q.; Peng, X.; Han, L.; Wang, X.; Wang, J.; Zeng, H. Recent advances in mechano-responsive hydrogels for biomedical applications. ACS Appl. Polym. Mater. 2020, 2, 1092–1107. [Google Scholar] [CrossRef]
- Praveen, V.K.; Vedhanarayanan, B.; Mal, A.; Mishra, R.K.; Ajayaghosh, A. Self-Assembled Extended π-Systems for Sensing and Security Applications. Acc. Chem. Res. 2020, 53, 496–507. [Google Scholar] [CrossRef]
- Sagara, Y.; Takahashi, K.; Nakamura, T.; Tamaoki, N. Mechanochromic Luminescence from crystals consisting of intermolecular hydrogen-bonded sheets. Chem. Asian J. 2020, 15, 478–482. [Google Scholar] [CrossRef] [Green Version]
- Isoda, K.; Matsubara, M.; Ikenaga, A.; Akiyama, Y.; Mutoh, Y. Reversibly/irreversibly stimuli-responsive inks based on N-heteroacene liquids. J. Mater. Chem. C 2019, 7, 14075–14079. [Google Scholar] [CrossRef]
- Seki, T.; Ida, K.; Ito, H. A meta-diisocyanide benzene-based aryl gold isocyanide complex exhibiting multiple solid-state molecular arrangements and luminescent mechanochromism. Mater. Chem. Front. 2018, 2, 1195–1200. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, A.; Kato, M. Stimuli-responsive luminescent copper(I) complexes for intelligent emissive devices. Chem. Lett. 2017, 46, 154–162. [Google Scholar] [CrossRef]
- Yamada, S.; Mitsuda, A.; Adachi, K.; Hara, M.; Konno, T. Development of light-emitting liquid-crystalline polymers with a pentafluorinated bistolane-based luminophore. New J. Chem. 2020, 44, 5684–5691. [Google Scholar] [CrossRef]
- Morita, M.; Yamada, S.; Agou, T.; Kubota, T.; Konno, T. Luminescence tuning of fluorinated bistolanes via electronic or aggregated-structure control. Appl. Sci. 2019, 9, 1905. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Miyano, K.; Agou, T.; Kubota, T.; Konno, T. 2-Chloroalkoxy-substituted pentafluorinated bistolanes as novel light-emitting liquid crystals. Crystals 2019, 9, 195. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Morita, M.; Konno, T. Multi-color photoluminescence induced by electron-density distribution of fluorinated bistolane derivatives. J. Fluor. Chem. 2017, 202, 54–64. [Google Scholar] [CrossRef]
- Yamada, S.; Miyano, K.; Konno, T.; Agou, T.; Kubota, T.; Hosokai, T. Fluorine-containing bistolanes as light-emitting liquid crystalline molecules. Org. Biomol. Chem. 2017, 15, 5949–5958. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Yamada, S.; Konno, T. Fluorine-induced emission enhancement of tolanes via formation of tight molecular aggregates. New J. Chem. 2020, 44, 6704–6708. [Google Scholar] [CrossRef]
- Yamada, S.; Nishizawa, A.; Morita, M.; Hosokai, T.; Okabayashi, Y.; Agou, T.; Hosoya, T.; Kubota, T.; Konno, T. Synthesis and characterization of bent fluorine-containing donor-π-acceptor molecules as intense luminophores with large Stokes shifts. Org. Biomol. Chem. 2019, 17, 6911–6919. [Google Scholar] [CrossRef]
- Grunwald, M.A.; Haenle, J.C.; Kreß, K.C.; Forschner, R.; Wöhrle, T.; Frey, W.; Giesselmann, F.; Laschat, S. Mesomorphic properties of cyanobiphenyl dimers with a central malonate unit. Liq. Cryst. 2018, 45, 1626–1636. [Google Scholar] [CrossRef]
- Arakawa, Y.; Komatsu, K.; Tsuji, H. Twist-bend nematic liquid crystals based on thioether linkage. New J. Chem. 2019, 43, 6786–6793. [Google Scholar] [CrossRef]
- Prabhu, R.; Yelamaggad, C.V. Structure-property correlations in cyanobiphenyl-based dimer-like mesogens. J. Phys. Chem. B 2015, 119, 11935–11952. [Google Scholar] [CrossRef]
- Arakawa, Y.; Kang, S.; Nakajima, S.; Sakajiri, K.; Cho, Y.; Kawauchi, S.; Watanabe, J.; Konishi, G.-I. Diphenyltriacetylenes: Novel nematic liquid crystal materials and analysis of their nematic phase-transition and birefringence behaviours. J. Mater. Chem. C 2013, 1, 8094–8102. [Google Scholar] [CrossRef]
- An, P.; Shi, Z.-F.; Dou, W.; Cao, X.-P.; Zhang, H.-L. Synthesis of 1,4-bis[2,2-bis(4-alkoxyphenyl)vinyl]benzenes and side chain modulation of their solid-state emission. Org. Lett. 2009, 12, 4364–4367. [Google Scholar] [CrossRef]
- Krause, M.; Ligneau, X.; Stark, H.; Garbarg, M.; Schwartz, J.-C.; Schunack, W. 4-Alkynylphenyl imidazolylpropyl ethers as selective histamine H3-receptor antagonists with high oral central nervous system activity. J. Med. Chem. 1998, 41, 4171–4176. [Google Scholar] [PubMed]
- Smeyanov, A.; Schmidt, A. K3PO4-KOH Mixture as efficient reagent for the deprotection of 4-aryl-2-methyl-3-butyn-2-ols to terminal acetylenes. Synth. Commun. 2013, 43, 2809–2816. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Okuno, K.; Shigeta, Y.; Kishi, R.; Nakano, M. Non-empirical tuning of CAM-B3LYP functional in time-dependent density functional theory for excitation energies of diarylethene derivatives. Chem. Phys. Lett. 2013, 585, 201–206. [Google Scholar] [CrossRef]
- Andzelm, J.; Kölmel, C.; Klamt, A. Incorporation of solvent effects into density functional calculations of molecular energies and geometries. J. Chem. Phys. 1995, 103, 9312–9320. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Lam, J.W.Y.; Li, J.; Chan, C.Y.K.; Chen, Y.; Zhao, N.; Han, T.; Tang, B.Z. Stoichiometric imbalance-promoted synthesis of polymers containing highly substituted naphthalenes: Rhodium-catalyzed oxidative polycoupling of arylboronic acids and internal diynes. Polym. Chem. 2013, 4, 1372–1380. [Google Scholar] [CrossRef] [Green Version]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Mondal, S.; Chatterjee, S.; Halder, R.; Jana, B.; Singh, C. Role of dispersive fluorous interaction in the solvation dynamics of the perfluoro group containing molecules. J. Phys. Chem. B 2017, 121, 7681–7688. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, J.; Yamaguchi, K.; Yamawaki, K.; Yasuda, T.; Nishimura, Y.; Kanbara, T. Modulation of the emission mode of a Pt(II) complex via intermolecular interactions. Inorg. Chem. 2017, 56, 8726–8729. [Google Scholar] [CrossRef] [PubMed]
- Pati, A.K.; Gharpure, S.J.; Mishra, A.K. White light emission in butadiyne bridged pyrene-phenyl hybdrid fluorophore: Understanding the photophysical importance of diyne spacer and utilizing the excited-state photophysics for vapor detection. J. Phys. Chem. A 2016, 120, 5838–5847. [Google Scholar] [CrossRef] [PubMed]







| Molecule | Dipole Moment (D) | HOMO (eV) | LUMO (eV) | Theoretical Transition 2 | Transition Energy (nm) 2 | f2,3 |
|---|---|---|---|---|---|---|
| 1b | 7.12 | −7.51 | −1.31 | HOMO → LUMO (91%) | 315 | 1.368 |
| 2a | 1.60 | −7.53 | −1.32 | HOMO–1 → LUMO (45%) HOMO → LUMO + 1 (45%) | 316 | 2.581 |
| 2b | 0 | −7.52 | −1.32 | HOMO–1 → LUMO (45%) HOMO → LUMO + 1 (45%) | 315 | 2.795 |
| Dyad | λabs [nm] (ε × 10−3 [L mol−1 cm−1]) 1 | λPL [nm] 2 (ΦPL) 3 |
|---|---|---|
| 2a | 321sh (59.7), 331 (60.7) | 417 (0.10) |
| 2b | 321sh (71.8), 330 (73.0) | 420 (0.10) |
| 2c | 321sh (67.9), 330 (68.9) | 420 (0.10) |
| 2d | 321sh (57.8), 330 (58.3) | 419 (0.09) |
| 2e | 321sh (57.7), 331 (58.5) | 419 (0.09) |
| 2f | 321sh (73.0), 329 (73.6) | 417 (0.09) |
| Dyad | States | λPL [nm] 1 (ΦPL) 2 |
|---|---|---|
| 2a | Crystalline | 427, 458 (0.17) |
| PMMA (1 wt % of 2a) | 406 (0.39) | |
| PMMA (20 wt % of 2a) | 423 (0.23) | |
| Ground | 432 (0.25) | |
| 2b | Crystalline | 366, 412 (0.41) |
| Ground | 418 (0.56) | |
| 2c | Crystalline | 427 (0.45) |
| 2d | Crystalline | 428, 460 (0.30) |
| Ground | 433, 458 (0.48) | |
| 2e | Crystalline | 420 (0.22) |
| 2f | Crystalline | 411 (0.42) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, S.; Uto, E.; Agou, T.; Kubota, T.; Konno, T. Fluorinated Tolane Dyads with Alkylene Linkage: Synthesis and Evaluation of Photophysical Characteristics. Crystals 2020, 10, 711. https://doi.org/10.3390/cryst10080711
Yamada S, Uto E, Agou T, Kubota T, Konno T. Fluorinated Tolane Dyads with Alkylene Linkage: Synthesis and Evaluation of Photophysical Characteristics. Crystals. 2020; 10(8):711. https://doi.org/10.3390/cryst10080711
Chicago/Turabian StyleYamada, Shigeyuki, Eiji Uto, Tomohiro Agou, Toshio Kubota, and Tsutomu Konno. 2020. "Fluorinated Tolane Dyads with Alkylene Linkage: Synthesis and Evaluation of Photophysical Characteristics" Crystals 10, no. 8: 711. https://doi.org/10.3390/cryst10080711
APA StyleYamada, S., Uto, E., Agou, T., Kubota, T., & Konno, T. (2020). Fluorinated Tolane Dyads with Alkylene Linkage: Synthesis and Evaluation of Photophysical Characteristics. Crystals, 10(8), 711. https://doi.org/10.3390/cryst10080711