Single Source Precursor for PAD-LaMnO3 Thin Films
Abstract
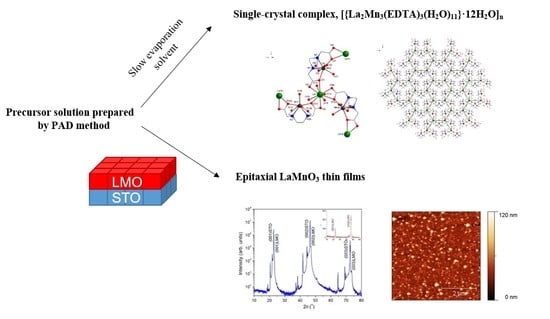
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Precursor Characterization
3.2. Single Crystal Characterization
3.3. LaMnO3 Thin Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khanduri, H.; Dimri, M.C.; Vasala, S.; Leinberg, S.; Lohmus, R.; Ashworth, T.V.; Mere, A.; Krustok, J.; Karppinen, M.; Stern, R. Magnetic and structural studies of LaMnO3 thin films prepared by atomic layer deposition. J. Phys. D Appl. Phys. 2013, 46, 46175003. [Google Scholar] [CrossRef]
- Zhu, X.; Lei, H.; Shi, D.; Zhang, L.; Wang, L.; Sun, Y.; Song, W.; Dou, S.; Yang, J.; Gu, H. Chemical Solution Deposition of LaMnO3 Buffer Layers for Coated Conductors. IEEE Trans. Appl. Supercond. 2007, 17, 3880–3885. [Google Scholar] [CrossRef] [Green Version]
- Aruta, C.; Angeloni, M.; Balestrino, G.; Boggio, N.G.; Medaglia, P.G.; Tebano, A. Preparation and characterization of LaMnO3 thin films grown by pulsed laser deposition. J. Appl. Phys. 2006, 100, 023910. [Google Scholar] [CrossRef]
- Gupta, A.; McGuire, T.R.; Duncombe, P.R.; Rupp, M.; Sun, J.Z.; Gallagher, W.J.; Xiao, G. Growth and giant magnetoresistance properties of La-deficient LaxMnO3−δ (0.67 ≤ x ≤ 1) films. Appl. Phys. Lett. 1995, 67, 3494. [Google Scholar] [CrossRef]
- Vila-Fungueiriño, J.M.; Rivas-Murias, B.; Rodríguez-González, B.; Txoperena, O.; Ciudad, D.; Hueso, L.E.; Lazzari, M.; Rivadulla, F. Room-Temperature Ferromagnetism in Thin Films of LaMnO3 Deposited by a Chemical Method Over Large Areas ACS Appl. Mat. Interfaces 2015, 7, 5410–5414. [Google Scholar] [CrossRef] [PubMed]
- Mlowe, S.; Nyamen, L.D.; Ndifon, P.T.; Malik, M.A.; Raftery, J.; O’Brien, P.; Revaprasadu, N. Aerosol assisted chemical vapor deposition (AACVD) of CdS thin films from heterocyclic cadmium (II) complexes. Ionrg. Chim. Acta 2015, 434, 181–187. [Google Scholar] [CrossRef]
- Shi, D.Q.; Zhu, X.B.; . Kim, J.H.; Lei, H.C.; Wang, L.; Sun, Y.P.; Zeng, R.; Dou, S.X. Chemical solution deposition of LaMnO3-based films for coated conductors. J. Phys. Conf. Ser. 2008, 97, 012054. [Google Scholar] [CrossRef]
- Gadani, K.; Keshvani, M.J.; Dhruv, D.; Boricha, H.; Rathod, K.N.; Prajapati, P.; Joshi, A.D.; Pandya, D.D.; Shah, N.A.; Solanki, P.S. Low field magnetoelectric and magnetotransport properties of sol–gel grown nanostructured LaMnO3 manganites. J. All. Comp. 2017, 719, 47–57. [Google Scholar] [CrossRef]
- Jia, Q.X.; McCleskey, T.M.; Burrell, A.K.; Lin, Y.; Collis, G.E.; Wang, H.; Li, A.D.Q.; Foltyn, S.R. Polymer-assisted deposition of metal-oxide films. Nat. Mater. 2004, 3, 529. [Google Scholar] [CrossRef]
- Mos, R.B.; Petrisor, T., Jr.; Nasui, M.; Calleja, A.; Puig, T.; Ciontea, L.; Petrisor, T. Enhanced structural and morphological properties of Gd-doped CeO2 thin films obtained by polymer-assisted deposition. Mater. Lett. 2014, 124, 306–309. [Google Scholar] [CrossRef]
- Stavila, V.; Davidovich, R.L.; Gulea, A.; Whitmire, K.H. Bismuth (III) complexes with aminopolycarboxylate and polyaminopolycarboxylate ligands: Chemistry and structure. Coord. Chem. Rev. 2006, 250, 2782. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Watcher, J.; O’Keefee, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 469, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cingolani, A.; Galli, S.; Masciocchi, N.; Pandolfo, L.; Pettinari, C.; Sironi, A. Sorption−Desorption Behavior of Bispyrazolato−Copper(II) 1D Coordination Polymers. J. Am. Chem. Soc. 2005, 127, 6144. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-C.; Hou, Y.-H.; Dong, X.; Yang, Y.-C.; Xia, W.-S.; Weng, W.; Zhou, Z.-H. Well-defined lanthanum ethylenediaminetetraacetates as the precursors of catalysts for the oxidative coupling of methane. Inorg. Chim. Acta 2015, 434, 221–229. [Google Scholar] [CrossRef]
- Yi, G.S.; Lu, H.C.; Zhao, S.Y.; Ge, Y.; Yang, W.J.; Chen, D.P.; Guo, L.H. Synthesis, Characterization, and Biological Application of Size-Controlled Nanocrystalline NaYF4:Yb,Er Infrared-to-Visible Up-Conversion Phosphors. Nano Lett. 2004, 4, 2191. [Google Scholar] [CrossRef]
- Liu, D.S.; Sui, Y.; Li, C.H.; Cheng, W.T.; Wang, T.W.; You, X.Z. Synthesis, structure and magnetic properties of a two-dimensional manganese(II) complex with a maximum denticity of ethylenediaminetetraacetic ligand. Inorg. Chim. Acta 2011, 376, 112–117. [Google Scholar] [CrossRef]
- Liu, D.S.; Qiu, Z.J.; Xiao, Y.L.; Dhen, Y.J.; Zhou, Q.; Chen, W.T.; Sui, Y. A novel tetranuclear Pb2+ compound based on ethylenediaminetetraacetate and azide mixed-ligands: Synthesis, structure and properties. J. Solid State Chem. 2019, 279, 120952. [Google Scholar] [CrossRef]
- Liu, D.S.; Qiu, Z.J.; Fu, X.; Liu, Y.Z.; Ding, P.; Zhou, Y.X.; Sui, Y. Synthesis, structures and properties of three lead coordination polymers based on ethylenediaminetetraacetate ligand. J. Solid State Chem. 2019, 278, 120879. [Google Scholar] [CrossRef]
- Xiong, D.B.; Chen, H.H.; Yang, X.X.; Zhao, J.T. Hydrothermal synthesis and characterization of a new 1-D polymeric lanthanum ethylenediaminetetraacetate with less metal-aqua coordination: {[La(EDTA)(H2O)]2}n. Inorg. Chim. Acta 2007, 360, 1616–1620. [Google Scholar] [CrossRef]
- Mos, R.B.; Petrisor, T.; Nasui, M.; Mesaros, A.; Gabor, M.S.; Senila, M.; Ware, E.; Ciontea, L.; Petrisor, T., Jr. Epitaxial La0.7Sr0.3MnO3 nanostructures obtained by polymer-assisted surface decoration (PASD). Mater. Lett. 2016, 171, 281–284. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillps, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Sheldrik, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- William, T.P. DIAMOND-Visual Crystal Structure Information System; Crystal Impact: Bonn, Germany, 2001. [Google Scholar]







| Compound | 1 |
|---|---|
| Empirical formula | C30H58La2Mn3N6O47 |
| Formula weight | 1697.46 |
| Temperature (K) | 297(2) |
| Wavelength (Å) | 0.71073 |
| Crystal system | Monoclinic |
| Space group | C2 |
| Unit cell dimensions | |
| a (Å) | 16.1227(17) |
| b (Å) | 14.8049(16) |
| c (Å) | 14.8736(16) |
| α (°) | 90 |
| β (°) | 116.107(2) |
| γ (°) | 90 |
| Volume (Å3) | 3188.0(6) |
| Z | 2 |
| Dc (mg/cm3) | 1.768 |
| Absorption coefficient (mm−1) | 2.000 |
| F(000) | 1690 |
| Crystal size (mm) | 0.30 × 0.26 × 0.23 |
| θ range for data collection (°) | 1.525 to 25.004 |
| Reflections collected | 15110 |
| Independent reflections | 5597 [R(int) = 0.0365] |
| Refinement method | Full matrix least-squares on F2 |
| Data/restraints/parameters | 5597/48/461 |
| Goodness-of-fit on F2 | 1.020 |
| Final R indices [I > 2σ(I)] | R1 = 0.0319, wR2 = 0.0797 |
| R indices (all data) | R1 = 0.0342, wR2 = 0.0811 |
| Absolute structure parameter | −0.011(8) |
| Largest diffraction peak and hole, eA−3 | 0.891 and −0.353 |
| Atoms 1, 2 | d 1,2 (Å) X-ray | d 1,2 (Å) FT-IR | Atoms 1, 2 | d 1,2 (Å) X-ray | d 1,2 (Å) FT-IR |
|---|---|---|---|---|---|
| La(1)–O(1) | 2.747(6) | 2.708 | Mn(1)–O(10) | 2.339(5) | 2.325 |
| La(1)–O(2) | 2.576(6) | 2.565 | Mn(1)–O(11) | 2.191(6) | 2.172 |
| La(1)–O(7a) | 2.755(5) | 2.733 | Mn(1)–O(18) | 2.137(10) | 2.119 |
| La(1)–O(8a) | 2.530(6) | 2.545 | Mn(1)–O(11b) | 2.191(6) | 2.172 |
| La(1)–O(9) | 2.521(7) | 2.551 | Mn(1)–O(10b) | 2.339(5) | 2.325 |
| La(1)–O(10) | 2.799(5) | 2.711 | Mn(1)–N(3) | 2.380(6) | 2.355 |
| La(1)–O(13) | 2.562(5) | 2.539 | Mn(1)–N(2b) | 2.380(6) | 2.291 |
| La(1)–O(14) | 2.569(6) | 2.551 | |||
| La(1)–O(15) | 2.529(6) | 2.544 | Mn(2)–O(1) | 2.360(6) | 2.389 |
| La(1)–O(16) | 2.522(6) | 2.548 | Mn(2)–O(3) | 2.187(6) | 2.159 |
| Mn(2)–O(5) | 2.200(6) | 2.298 | |||
| Mn(2)–O(7) | 2.347(5) | 2.345 | |||
| Mn(2)–O(17) | 2.136(8) | 2.167 | |||
| Mn(2)–N(1) | 2.374(6) | 2.305 | |||
| Mn(2)–N(2) | 2.338(7) | 2.371 |
| D–H···A | Type | d(D–H) (Å) | d(H···A) (Å) | d(D···A) (Å) | <(DHA) (°) |
|---|---|---|---|---|---|
| O(14)–H(14D)···O(5) | intra | 0.85(4) | 1.96(3) | 2.786(8) | 164(9) |
| O(15)–H(15D)···O(3a) | intra | 0.86(6) | 1.96(7) | 2.802(9) | 167(12) |
| O(16)–H(16C)···O(11) | intra | 0.86(6) | 1.90(9) | 2.720(9) | 161(11) |
| O(17)–H(17C)···O(8a) | intra | 0.84(5) | 1.88(7) | 2.626(11) | 146(14) |
| O(17)–H(17D)···O(9a) | intra | 0.86(5) | 1.87(6) | 2.712(10) | 165(6) |
| O(18)–H(18D)···O(2) | intra | 0.85(6) | 2.00(7) | 2.765(7) | 150(7) |
| O(13)–H(13C)..O(6d) | inter | 0.84(6) | 2.05(6) | 2.864(11) | 162(7) |
| O(15)–H(15C)..O(4c) | inter | 0.85(9) | 1.82(9) | 2.628(12) | 159(8) |
| O(16)–H(16D)..O(4c) | inter | 0.85(7) | 1.89(8) | 2.691(12) | 157(9) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonher, R.B.; Varga, R.A.; Nasui, M.; Jr, T.P.; Gabor, M.S.; Senila, M.; Rufoloni, A.; Petrisor, T.; Ciontea, L. Single Source Precursor for PAD-LaMnO3 Thin Films. Crystals 2020, 10, 851. https://doi.org/10.3390/cryst10090851
Sonher RB, Varga RA, Nasui M, Jr TP, Gabor MS, Senila M, Rufoloni A, Petrisor T, Ciontea L. Single Source Precursor for PAD-LaMnO3 Thin Films. Crystals. 2020; 10(9):851. https://doi.org/10.3390/cryst10090851
Chicago/Turabian StyleSonher, Ramona Bianca, Richard Attila Varga, Mircea Nasui, Traian Petrisor Jr, Mihai Sebastian Gabor, Marin Senila, Alessandro Rufoloni, Traian Petrisor, and Lelia Ciontea. 2020. "Single Source Precursor for PAD-LaMnO3 Thin Films" Crystals 10, no. 9: 851. https://doi.org/10.3390/cryst10090851
APA StyleSonher, R. B., Varga, R. A., Nasui, M., Jr, T. P., Gabor, M. S., Senila, M., Rufoloni, A., Petrisor, T., & Ciontea, L. (2020). Single Source Precursor for PAD-LaMnO3 Thin Films. Crystals, 10(9), 851. https://doi.org/10.3390/cryst10090851