Atmospheric Carbon Dioxide Capture as Carbonate into a Luminescent Trinuclear Cd(II) Complex with Tris(2-aminoethyl)amine Tripodal Ligand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Physical Measurements
2.2.1. X-ray Structure Determination
2.2.2. Spectroscopy
3. Results
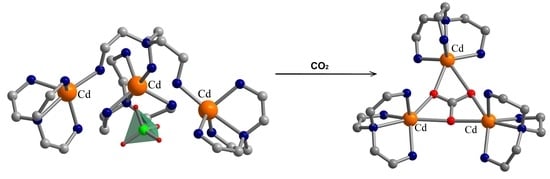
3.1. Description of the Crystal Structures
3.2. Spectral Properties
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palmer, D.A.; Van Eldik, R. The chemistry of Metal Carbonato and Carbon Dioxide Complexes. Chem. Rev. 1983, 83, 651–731. [Google Scholar] [CrossRef]
- Sołtys-Brzostek, K.; Terlecki, M.; Sokołowski, K.; Lewiński, J. Chemical fixation and conversion of CO2 into cyclic and cage-type metal carbonates. Coord. Chem. Rev. 2017, 334, 199–231. [Google Scholar] [CrossRef]
- English, N.J.; El-Hendawy, M.M.; Mooney, D.A.; MacElroy, J.M.D. Perspectives on atmospheric CO2 fixation in inorganic and biomimetic structures. Coord. Chem. Rev. 2014, 269, 85–95. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.-Y.; Jin, J.; Liu, X.-M.; Xue, G.-L. Layer structural bimetallic metamagnets obtained from the aggregation of Ru2(CO3)43− and Co2+ in existence of halogen. Cryst. Eng. Comm. 2013, 15, 4280–4287. [Google Scholar] [CrossRef]
- Khan, S.; Roy, S.; Bhar, K.; Kumar, R.K.; Maji, T.K.; Ghosh, B.K. Syntheses, structures and properties of μ3-carbonato bridged trinuclear zinc(II) complexes containing a tailored tetradentate amine. Polyhedron 2012, 32, 54–59. [Google Scholar] [CrossRef]
- Ghionoiu, A.-E.; Popescu, D.-L.; Maxim, C.; Madalan, A.M.; Haiduc, I.; Andruh, M. Atmospheric CO2 capture by a triphenyltin–1,2-bis(4-pyridyl)ethane system with formation of a rare trinuclear carbonato-centered core. Inorg. Chem. Commun. 2015, 58, 71–73. [Google Scholar] [CrossRef]
- Peng, Y.-X.; Xu, F.; Yin, G.; Liu, Q.; Huang, W. Three trinuclear copper(II) complexes bridged by μ3-CO32− with different coordination modes. J. Coord. Chem. 2012, 65, 3949–3959. [Google Scholar] [CrossRef]
- Kolks, G.; Lippard, S.J.; Waszczak, J.V. A Tricopper(II) Complex Containing a Triply Bridging Carbonate Group. J. Am. Chem. Soc. 1980, 102, 4832–4833. [Google Scholar] [CrossRef]
- Guo, Y.-N.; Chen, X.-H.; Xue, S.; Tang, J. Molecular Assembly and Magnetic Dynamics of Two Novel Dy6 and Dy8 Aggregates. Inorg. Chem. 2012, 51, 4035–4042. [Google Scholar] [CrossRef]
- Janzen, D.E.; Botros, M.E.; Van Derveer, D.G.; Grant, G.J. Fixation of atmospheric carbon dioxide by a cadmium(II) macrocyclic complex. Dalton Trans. 2007, 5316–5321. [Google Scholar] [CrossRef]
- Liang, X.; Parkinson, J.A.; Parsons, S.; Weishäupl, M.; Sadler, P.J. Cadmium Cyclam Complexes: Interconversion of Cis and Trans Configurations and Fixation of CO2. Inorg. Chem. 2002, 41, 4539–4547. [Google Scholar] [CrossRef] [PubMed]
- Bag, P.; Dutta, S.; Biswas, P.; Maji, S.K.; Flörke, U.; Nag, K. Fixation of carbon dioxide by macrocyclic lanthanide(III) complexes under neutral conditions producing self-assembled trimeric carbonato-bridged compounds with μ3-η2:η2:η2 bonding. Dalton Trans. 2012, 41, 3414–3423. [Google Scholar] [CrossRef]
- Liu, H.-X.; Zhang, X.; Gao, X.-J.; Chen, C.; Huang, D. Synthesis and mechanism study of a dimeric tetranuclear carbonate-bridged copper(II) complex resulting from CO2 fixation by controlling O2 concentration. Inorg. Chem. Commun. 2016, 68, 63–67. [Google Scholar] [CrossRef]
- Escuer, A.; El Fallah, M.S.; Kumar, S.B.; Mautner, F.; Vicente, R. Synthesis, crystal structure and magnetic behaviour of (μ3-CO3)[Ni3(Medpt)3(NCSe)4], a new example of trinuclear nickel(II) complex with pentadentate carbonato bridge and strong antiferromagnetic coupling. Polyhedron 1998, 18, 377–381. [Google Scholar] [CrossRef]
- Liu, C.-M.; Hao, X.; Zhang, D.-Q. CO2-fixation into carbonate anions for the construction of 3d-4f cluster complexes with salen-type Schiff base ligands: From molecular magnetic refrigerants to luminescent single-molecule magnets. Appl. Organomet. Chem. 2020, 5893, 1–14. [Google Scholar] [CrossRef]
- Escuer, A.; Vicente, R.; Kumar, S.J.; Mautner, F.A. Spin frustration in the butterfly-like tetrameric [Ni4(μ-CO3)2(aetpy)8]-[ClO4]4 [aetpy = (2-aminoethyl)pyridine] complex. Structure and magnetic properties. J. Chem. Soc. Dalton Trans. 1998, 3473–3477. [Google Scholar] [CrossRef]
- Marvaud, V.; Decroix, C.; Scuiller, A.; Guyard-Duhayon, C.; Vaissermann, J.; Gonnet, F.; Verdaguer, M. Hexacyanometalate Molecular Chemistry: Heptanuclear Heterobimetallic Complexes; Control of the Ground Spin State. Chem. Eur. J. 2003, 9, 1677–1691. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Gao, Y.; Qiu, L.-J.; Liu, B.-S.; Zhang, X.-R.; Cui, L.-L.; Yang, F. Synthesis and characterization of nickel(II) and copper(II) tricyanomethanide complexes with tris(2-aminoethyl)amine as co-ligand. Inorg. Chim. Acta 2014, 416, 215–221. [Google Scholar] [CrossRef]
- Pérez-Toro, I.; Domínguez-Martín, A.; Choquesillo-Lazarte, D.; Vílchez-Rodríguez, E.; Castiñeiras, A.; Niclós-Gutiérrez, J. Synthesis, thermogravimetric study and crystal structure of an N-rich copper(II) compound with tren ligands and nitrate counter-anions. Thermochim. Acta 2014, 593, 7–11. [Google Scholar] [CrossRef]
- Klüfers, P.; Mayer, P. A Star-Shaped Heptanuclear Tetramine Cadmium(II) complex. Acta Cryst. 1998, 54, 722–725. [Google Scholar] [CrossRef]
- Septavaux, J.; Tosi, C.; Jame, P.; Nervi, C.; Gobetto, R.; Leclaire, J. Simultaneous CO2 capture and metal purification from waste streams using triple-level dynamic combinatorial chemistry. Nat. Chem. 2020, 12, 202–212. [Google Scholar] [CrossRef] [PubMed]






| Compound | 1 | 2 |
|---|---|---|
| Chemical formula | C24H76Cd3Cl6N16O26 | C43H126Cd6Cl10N28O43 |
| M (g mol−1) | 1554.90 | 2752.61 |
| Temperature, (K) | 293 (2) | 293 (2) |
| Wavelength, (Å) | 0.71073 | 0.71073 |
| Crystal system | Triclinic | Monoclinic |
| Space group | P-1 | P21 |
| a (Å) | 14.8621 (7) | 17.4973 (6) |
| b (Å) | 15.3643 (6) | 12.2107 (4) |
| c (Å) | 15.5852 (8) | 23.5653 (8) |
| α (°) | 99.667 (4) | 90 |
| β (°) | 96.885 (4) | 100.059 (3) |
| γ (°) | 116.107 (4) | 90 |
| V (Å3) | 3073.9 (3) | 4957.4 (3) |
| Z | 2 | 2 |
| Dc (g cm−3) | 1.680 | 1.844 |
| μ (mm−1) | 1.372 | 1.629 |
| F (000) | 1572 | 2764 |
| Goodness-of-fit on F2 | 1.023 | 1.046 |
| Final R1, wR2 [I > 2σ(I)] | 0.0806, 0.2320 | 0.0523, 0.1404 |
| R1, wR2 (all data) | 0.0960, 0.2529 | 0.0598, 0.1473 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mădălan, A.M. Atmospheric Carbon Dioxide Capture as Carbonate into a Luminescent Trinuclear Cd(II) Complex with Tris(2-aminoethyl)amine Tripodal Ligand. Crystals 2021, 11, 1480. https://doi.org/10.3390/cryst11121480
Mădălan AM. Atmospheric Carbon Dioxide Capture as Carbonate into a Luminescent Trinuclear Cd(II) Complex with Tris(2-aminoethyl)amine Tripodal Ligand. Crystals. 2021; 11(12):1480. https://doi.org/10.3390/cryst11121480
Chicago/Turabian StyleMădălan, Augustin M. 2021. "Atmospheric Carbon Dioxide Capture as Carbonate into a Luminescent Trinuclear Cd(II) Complex with Tris(2-aminoethyl)amine Tripodal Ligand" Crystals 11, no. 12: 1480. https://doi.org/10.3390/cryst11121480
APA StyleMădălan, A. M. (2021). Atmospheric Carbon Dioxide Capture as Carbonate into a Luminescent Trinuclear Cd(II) Complex with Tris(2-aminoethyl)amine Tripodal Ligand. Crystals, 11(12), 1480. https://doi.org/10.3390/cryst11121480