TiO2 Modified Geopolymers for the Photocatalytic Dye Decomposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Methods
2.4. Photocatalytic Activity
3. Results and Discussion
3.1. Characterization of the Studied Materials
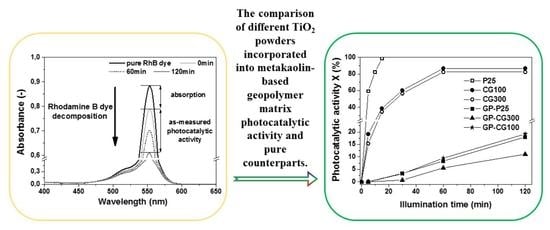
3.2. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Material | SiO2 | Al2O3 | CaO | MgO | Fe2O3 | TiO2 | K2O | SO3 | LOI * |
|---|---|---|---|---|---|---|---|---|---|
| Mefisto L05 | 52.34 | 41.54 | 0.13 | 0.02 | 0.98 | 1.46 | 0.48 | 0.11 | 2.41 |
| BFS | 20.79 | 7.36 | 39.78 | 3.21 | 1.90 | 0.44 | 1.63 | 10.11 | 11.70 |
| Particle Size (% of Total) | 2 [µm] | 5 [µm] | 8 [µm] | 10 [µm] | 15 [µm] | 25 [µm] | 45 [µm] | d10 [µm] | d50 [µm] | d90 [µm] |
|---|---|---|---|---|---|---|---|---|---|---|
| Mefisto L05 | 27.03 | 57.98 | 77.70 | 88.49 | 99.14 | 100.00 | 100.00 | 1.00 | 3.94 | 10.43 |
| BFS | 21.26 | 49.94 | 69.94 | 78.98 | 91.44 | 98.89 | 100.00 | 1.06 | 5.01 | 14.23 |
| Material | TiO2 | SiO2 | Al2O3 | Cl | P2O5 | Fe2O3 | Nb2O5 | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| CG100 | 93.49 | 0.12 | 0.03 | 0.00 | 0.05 | 0.03 | 0.25 | 1.36 | 4.61 |
| CG300 | 91.19 | 0.06 | 0.05 | 0.00 | 0.15 | 0.02 | 0.14 | 1.22 | 7.10 |
| P25 | 97.62 | 0.00 | 0.00 | 0.12 | 0.00 | 0.01 | 0.00 | 0.00 | 2.21 |
| Material | Average Particle Size [nm] * | Specific Surface Area (BET) [m2/g] | Mineralogical Composition |
|---|---|---|---|
| CG100 | 20/18 | 70–110 | Anatase |
| CG300 | 10/6 | 250–350 | Anatase |
| P25 | 21/20 | 40–60 | Anatase/rutile |
| Particle Size (% of Total) | 5 [µm] | 45 [µm] | 90 [µm] | 180 [µm] | 400 [µm] | d10 [µm] | d50 [µm] | d90 [µm] |
|---|---|---|---|---|---|---|---|---|
| GP | 23.96 | 78.54 | 93.48 | 99.84 | 100.00 | 2.10 | 17.14 | 74.72 |
| GP-CG100 | 22.88 | 66.67 | 82.60 | 92.90 | 100.00 | 2.09 | 21.97 | 141.96 |
| GP-CG300 | 24.50 | 72.39 | 86.34 | 94.18 | 100.00 | 2.07 | 18.37 | 117.09 |
| GP-P25 | 29.23 | 75.02 | 86.11 | 92.89 | 100.00 | 1.72 | 14.06 | 129.41 |
| Sample | Line | wt.% | wt.% | wt.% | wt.% | Average | SD |
|---|---|---|---|---|---|---|---|
| GP-CG100 | Ti Kα | 1.56 | 1.32 | 1.50 | 1.66 | 1.57 | 0.19 |
| GP-CG300 | Ti Kα | 1.66 | 1.68 | 1.62 | 1.32 | 1.57 | 0.17 |
| GP-P25 | Ti Kα | 1.80 | 1.56 | 1.58 | 1.63 | 1.64 | 0.11 |



References
- Harper, C.; Snowden, M. Environment and Society: Human Perspectives on Environmental Issues, 6th ed.; Taylor & Francis: New York, NY, USA, 2017. [Google Scholar]
- Frank, S.N.; Bard, A.J. Heterogeneous Photocatalytic Oxidation of Cyanide and Sulfite in Aqueous-Solutions at Semiconductor Powders. J. Phys. Chem. 1977, 81, 1484–1488. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Kennedy, D.R.; Ritchie, M.; Mackenzie, J. The Photosorption of Oxygen and Nitric Oxide on Titanium Dioxide. Trans. Faraday Soc. 1958, 54, 119–129. [Google Scholar] [CrossRef]
- Reiche, H.; Dunn, W.W.; Bard, A.J. Heterogeneous Photocatalytic and Photosynthetic Deposition of Copper on TiO2 and WO3 Powders. J. Phys. Chem. 1979, 83, 2248–2251. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.T.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. Synthesis and characterization of solar photoactive TiO2 nanoparticles with enhanced structural and optical properties. Adv. Powder Technol. 2018, 29, 211–219. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.G.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Zhang, Q.H. Effects of amorphous contents and particle size on the photocatalytic properties of TiO2 nanoparticles. Scr. Mater. 2001, 44, 1195–1198. [Google Scholar] [CrossRef]
- Jang, H.D.; Kim, S.K.; Kim, S.J. Effect of particle size and phase composition of titanium dioxide nanoparticles on the photocatalytic properties. J. Nanopart. Res. 2001, 3, 141–147. [Google Scholar] [CrossRef]
- Li, D.Q.; Song, H.C.; Meng, X.; Shen, T.T.; Sun, J.; Han, W.J.; Wang, X.K. Effects of Particle Size on the Structure and Photocatalytic Performance by Alkali-Treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Chunping, G.; Qiannan, W.; Jintao, L.; Wei, S. The effect of nano TiO2 on the durability of ultra-high-performance concrete with and without a flexural load. Ceramics 2018, 62, 374–381. [Google Scholar]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Shilova, O.A.; Vlasov, D.Y.; Zelenskaya, M.S.M.S.; Ryabusheva, Y.V.; Khamova, T.V.; Glebova, I.B.I.B.; Sinelnikov, A.A.; Marugin, A.M.; Frank-Kamenetskaya, O.V. Sol-Gel Derived TiO2 and Epoxy-Titanate Protective Coatings: Structure, Property, Fungicidal Activity and Biomineralization Effects. In Processes and Phenomena on the Boundary between Biogenic and Abiogenic Nature; Frank-Kamenetskaya, O.V., Panova, E.G., Lessovaia, S.N., Eds.; Springer: Cham, Switzerland, 2020; pp. 619–638. [Google Scholar]
- Dong, H.R.; Zeng, G.M.; Tang, L.; Fan, C.Z.; Zhang, C.; He, X.X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Pawar, M.; Sendogdular, S.T.; Gouma, P. A Brief Overview of TiO2 Photocatalyst for Organic Dye Remediation: Case Study of Reaction Mechanisms Involved in Ce-TiO2 Photocatalysts System. J. Nanomater. 2018, 2018, 5953609. [Google Scholar] [CrossRef] [Green Version]
- Miklecic, J.; Blagojevic, S.L.; Petric, M.; Jirous-Rajkovic, V. Influence of TiO2 and ZnO nanoparticles on properties of waterborne polyacrylate coating exposed to outdoor conditions. Prog. Org. Coat. 2015, 89, 67–74. [Google Scholar] [CrossRef]
- Ren, Y.A.; Chen, M.; Zhang, Y.; Wu, L.M. Fabrication of Rattle-Type TiO2/SiO2 Core/Shell Particles with Both High Photoactivity and UV-Shielding Property. Langmuir 2010, 26, 11391–11396. [Google Scholar] [CrossRef] [PubMed]
- Shandilya, N.; Le Bihan, O.; Bressot, C.; Morgeneyer, M. Emission of Titanium Dioxide Nanoparticles from Building Materials to the Environment by Wear and Weather. Environ. Sci. Technol. 2015, 49, 2163–2170. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B-Environ. 2015, 176, 396–428. [Google Scholar] [CrossRef] [Green Version]
- Zailan, S.N.; Mahmed, M.; Abdullah, M.; Sandu, A. Self-cleaning geopolymer concrete—A review. IOP Conf. Ser. Mater. Sci. Eng. 2016, 133, 012026. [Google Scholar] [CrossRef]
- Dundar, I.; Krichevskaya, M.; Katerski, A.; Acik, I.O. TiO2 thin films by ultrasonic spray pyrolysis as photocatalytic material for air purification. Roy Soc. Open Sci. 2019, 6, 181578. [Google Scholar]
- Malnieks, K.; Mezinskis, G.; Pavlovska, I. Effect of different dip-coating techniques on TiO2 thin film properties. Key Eng. Mater. 2017, 721, 128–132. [Google Scholar] [CrossRef]
- Masiala, T.M.; Bantu, A.K.M.; Bakambo, G.E.; Lunguya, J.M.; Kanza, J.L.K.; Muamba, O.M. Influence of pH Preparation on the Photo-Response of Electrodeposited Titanium Dioxide (TiO2) Thin Films. Int. J. Mater. Sci. Appl. 2019, 5, 207–213. [Google Scholar]
- Pala, L.P.R.; Uday, V.; Gogoi, D.; Peela, N.R. Surface and photocatalytic properties of TiO2 thin films prepared by non-aqueous surfactant assisted sol-gel method. J. Environ. Chem. Eng. 2020, 8, 104267. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Wu, Y.G.; Lu, B.W.; Bai, T.; Wang, H.; Du, F.P.; Zhang, Y.F.; Cai, L.; Jiang, C.; Wang, W.J. Geopolymer, green alkali activated cementitious material: Synthesis, applications and challenges. Constr. Build. Mater. 2019, 224, 930–949. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Davidovits, J. Geopolymer Chemistry and Applications, 5th ed.; Institut Géopolymère: Saint-Quentin, France, 2020. [Google Scholar]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Jin, M.T.; Zheng, Z.D.; Sun, Y.; Chen, L.W.; Jin, Z.F. Resistance of metakaolin-MSWI fly ash based geopolymer to acid and alkaline environments. J. Non-Cryst. Solids 2016, 450, 116–122. [Google Scholar] [CrossRef]
- Karthik, A.; Sudalaimani, K.; Vijayakumar, C.T. Durability study on coal fly ash-blast furnace slag geopolymer concretes with bio-additives. Ceram. Int. 2017, 43, 11935–11943. [Google Scholar] [CrossRef]
- Lahoti, M.K.; Tan, K.H.; Yang, E.H. A critical review of geopolymer properties for structural fire-resistance applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Neupane, K.; Chalmers, D.; Kidd, P. High-Strength Geopolymer Concrete—Properties. Advant. Chall. Adv. Mater. 2018, 7, 15–25. [Google Scholar]
- Rasaki, S.A.; Zhang, B.X.; Guarecuco, R.; Thomas, T.; Yang, M.H. Geopolymer for use in heavy metals adsorption, and advanced oxidative processes: A critical review. J. Clean. Prod. 2019, 213, 42–58. [Google Scholar] [CrossRef]
- Novotná, M.; Perná, I.; Hanzlíček, T. Review of Possible Fillers and Additives for Geopolymer Materials. Wasteforum 2020, 8, 78–89. [Google Scholar]
- Duan, P.; Yan, C.J.; Luo, W.J.; Zhou, W. Effects of adding nano-TiO2 on compressive strength, drying shrinkage, carbonation and microstructure of fluidized bed fly ash based geopolymer paste. Constr. Build. Mater. 2016, 106, 115–125. [Google Scholar] [CrossRef]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J.J. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef]
- Baranowska-Wojcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—A Review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Czajka, M.; Sawicki, K.; Sikorska, K.; Popek, S.; Kruszewski, M.; Kapka-Skrzypczak, L. Toxicity of titanium dioxide nanoparticles in central nervous system. Toxicol. Vitr. 2015, 29, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Navratil, T.; Kacerova, T.; Zamostna, B.; Fenclova, Z.; Vlckova, S.; Kacer, P. NanoTiO2 Sunscreen Does Not Prevent Systemic Oxidative Stress Caused by UV Radiation and a Minor Amount of NanoTiO2 is Absorbed in Humans. Nanomaterials 2019, 9, 888. [Google Scholar] [CrossRef] [Green Version]
- Wattanasiriwech, D.; Yomthong, K.; Wattanasiriwech, S. Adsorption efficiency and photocatalytic activity of fly ash-based geopolymer foam mortar. Ceram. Int. 2021, 47, 27361–27371. [Google Scholar] [CrossRef]
- Kaya-Ozkiper, K.; Uzun, A.; Soyer-Uzun, S. Red mud- and metakaolin-based geopolymers for adsorption and photocatalytic degradation of methylene blue: Towards self-cleaning construction materials. J. Clean. Prod. 2021, 288, 125120. [Google Scholar] [CrossRef]
- Longhi, M.A.; Zhang, Z.H.; Rodriguez, E.D.; Kirchheim, A.P.; Wang, H. Efflorescence of Alkali-Activated Cements (Geopolymers) and the Impacts on Material Structures: A Critical Analysis. Front. Mater. 2019, 6, 89. [Google Scholar] [CrossRef]
- Perná, I.; Hanzlíček, T.; Ertl, Z. Utilization of biomass ashes for construction purposes. In Proceedings of the Third Euro Mediterranean Symposium in Advances on Geomaterials and Structures, Tunis, Tunisia, 1 January 2010; Darve, F., Doghri, I., El Fatmi, R., Hassis, H., Zenzri, H., Eds.; LGC-ENIT: Tunis, Tunisia, 2010; pp. 661–666. [Google Scholar]
- Perna, I.; Hanzlicek, T. The setting time of a clay-slag geopolymer matrix: The influence of blast-furnace-slag addition and the mixing method. J Clean. Prod. 2016, 112, 1150–1155. [Google Scholar] [CrossRef]
- Hanzlicek, T.; Perna, I.; Ulicna, K.; Rimal, V.; Stepankova, H. The Evaluation of Clay Suitability for Geopolymer Technology. Minerals 2020, 10, 852. [Google Scholar] [CrossRef]
- Srb, M.; Milasheuskaya, Y.; Jambor, R.; Kopecká, K.; Knotek, P. Low-Temperature Sn0 Nanoparticles Synthesis by Means of Tin (II) N,N-Complexes Reduction. ChemistrySelect 2021, 6, 3926–3931. [Google Scholar] [CrossRef]
- EVA, Diffracplus Basic Evaluating Package, version 19; Bruker AXS GmbH: Karlsruhe, Germany, 2013.
- DIFRAC. EVA, version 2.1; Bruker AXS GmbH: Karlsruhe, Germany, 2011. [Google Scholar]
- Joint Committee on Powder Diffraction Standards. International Centre of Diffraction Data; ICDD: Swarthmore, PA, USA, 2021. [Google Scholar]
- Knotek, P.; Kutalek, P.; Cernoskova, E.; Tichy, L.; Janicek, P. The wettability of variously treated AA42Se58 thin films. Mater. Chem. Phys. 2019, 221, 216–223. [Google Scholar] [CrossRef]
- Maiti, M.; Sarkar, M.; Maiti, S.; Malik, M.A.; Xu, S.L. Modification of geopolymer with size controlled TiO2 nanoparticle for enhanced durability and catalytic dye degradation under UV light. J. Clean. Prod. 2020, 255, 120183. [Google Scholar] [CrossRef]
- Jiang, X.Z.; Manawan, M.; Feng, T.; Qian, R.F.; Zhao, T.; Zhou, G.D.; Kong, F.T.; Wang, Q.; Dai, S.Y.; Pan, J.H. Anatase and rutile in evonik aeroxide P25: Heterojunctioned or individual nanoparticles? Catal. Today 2018, 300, 12–17. [Google Scholar] [CrossRef]
- PRECHEZA a.s. Available online: https://www.precheza.cz/en/history (accessed on 3 July 2021).
- Tsai, W.-B.; Kao, J.-Y.; Wu, T.-M.; Cheng, W.-T. Dispersion of Titanium Oxide Nanoparticles in Aqueous Solution with Anionic Stabilizer via Ultrasonic Wave. J. Nanopart. 2016, 2016, 6539581. [Google Scholar] [CrossRef]
- Sentein, C.; Guizard, B.; Giraud, S.; Yé, C.; Ténégal, F. Dispersion and stability of TiO2 nanoparticles synthesized by laser pyrolysis in aqueous suspensions. J. Phys. Conf. Ser. 2009, 170, 012013. [Google Scholar] [CrossRef] [Green Version]
- Falah, M.; MacKenzie, K.J.D. Photocatalytic Nanocomposite Materials Based on Inorganic Polymers (Geopolymers): A Review. Catalysts 2020, 10, 1158. [Google Scholar] [CrossRef]
- Chan, C.K.; Porter, J.F.; Li, Y.G.; Guo, W.; Chan, C.M. Effects of calcination on the microstructures and photocatalytic properties of nanosized titanium dioxide powders prepared by vapor hydrolysis. J. Am. Ceram. Soc. 1999, 82, 566–572. [Google Scholar] [CrossRef]
- Porter, J.F.; Li, Y.G.; Chan, C.K. The effect of calcination on the microstructural characteristics and photoreactivity of Degussa P-25 TiO2. J. Mater. Sci. 1999, 34, 1523–1531. [Google Scholar] [CrossRef]
- Sanalkumar, K.U.A.; Yang, E.H. Self-cleaning performance of nano-TiO2 modified metakaolin-based geopolymers. Cem. Concr. Comp. 2021, 115, 103847. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, J.C.; Hidaka, H. Highly selective deethylation of rhodamine B: Adsorption and photooxidation pathways of the dye on the TiO2/SiO2 composite photocatalyst. Int. J. Photoenergy 2003, 5, 209. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.S.; Wang, D.W. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef] [Green Version]









| Mix Designation | Content [g] | ||||||
|---|---|---|---|---|---|---|---|
| Mefisto L05 | BFS | Alkaline Solution | Water | TiO2 CG100 | TiO2 CG300 | TiO2 P25 | |
| GP | 100 | 33 | 120 | 20 | - | - | - |
| GP-CG100 | 100 | 33 | 120 | 20 | 5 | - | - |
| GP-CG300 | 100 | 33 | 120 | 20 | - | 5 | - |
| GP-P25 | 100 | 33 | 120 | 20 | - | - | 5 |
| Sample Name | GP | GP-CG100 | GP-CG300 | GP-P25 |
|---|---|---|---|---|
| Ti (wt.%) | 0.61 ± 0.06 | 1.57 ± 0.19 | 1.57 ± 0.17 | 1.64 ± 0.11 |
| Material | SiO2 | Al2O3 | CaO | MgO | Fe2O3 | TiO2 | K2O | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| GP | 40.75 | 18.56 | 6.48 | 0.34 | 1.42 | 0.94 | 13.98 | 1.35 | 15.53 |
| GP-CG100 | 39.57 | 17.90 | 6.31 | 0.31 | 1.40 | 3.42 | 13.91 | 1.34 | 15.19 |
| GP-CG300 | 39.60 | 18.12 | 6.50 | 0.36 | 1.42 | 2.93 | 13.87 | 1.33 | 15.20 |
| GP-P25 | 38.62 | 17.81 | 6.04 | 0.35 | 1.35 | 3.31 | 13.32 | 1.33 | 17.30 |
| Sample | Peak Area | Area Ratio | Amorphous Phase Proportion, wt.% | |||
|---|---|---|---|---|---|---|
| (101) Anatase | (101) Quartz | (104) Calcite | Anatase/ Quartz | Anatase/ Calcite | ||
| GP | 2.2 | 1.7 | 3.3 | 1.3 | 0.7 | 66–69 |
| GP-P25 | 10.3 | 2.4 | 3.8 | 4.3 | 2.7 | 63–65 |
| GP-CG100 | 10.5 | 2.2 | 3.1 | 4.8 | 3.4 | 62–64 |
| GP-CG300 | 2.6 | 2.1 | 3.1 | 1.2 | 0.8 | 67–69 |
| Sample | k (min−1) | R2 |
|---|---|---|
| P25 | 0.27 | 0.981 |
| GC100 | 0.033 | 0.998 |
| GC300 | 0.029 | 0.999 |
| MM-GP-P25 (parent GP + 3 wt.% of P25) | 0.0012 | 0.990 |
| GP-P25 | 0.0018 | 0.997 |
| GP-CG100 | 0.0017 | 0.996 |
| GP-CG300 | 0.0010 | 0.984 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novotná, M.; Knotek, P.; Hanzlíček, T.; Kutálek, P.; Perná, I.; Melánová, K.; Černošková, E.; Kopecká, K. TiO2 Modified Geopolymers for the Photocatalytic Dye Decomposition. Crystals 2021, 11, 1511. https://doi.org/10.3390/cryst11121511
Novotná M, Knotek P, Hanzlíček T, Kutálek P, Perná I, Melánová K, Černošková E, Kopecká K. TiO2 Modified Geopolymers for the Photocatalytic Dye Decomposition. Crystals. 2021; 11(12):1511. https://doi.org/10.3390/cryst11121511
Chicago/Turabian StyleNovotná, Martina, Petr Knotek, Tomáš Hanzlíček, Petr Kutálek, Ivana Perná, Klára Melánová, Eva Černošková, and Kateřina Kopecká. 2021. "TiO2 Modified Geopolymers for the Photocatalytic Dye Decomposition" Crystals 11, no. 12: 1511. https://doi.org/10.3390/cryst11121511
APA StyleNovotná, M., Knotek, P., Hanzlíček, T., Kutálek, P., Perná, I., Melánová, K., Černošková, E., & Kopecká, K. (2021). TiO2 Modified Geopolymers for the Photocatalytic Dye Decomposition. Crystals, 11(12), 1511. https://doi.org/10.3390/cryst11121511